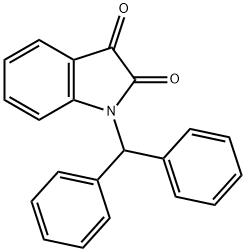
1-benzhydrylindoline-2,3-dione synthesis
- Product Name:1-benzhydrylindoline-2,3-dione
- CAS Number:94878-41-8
- Molecular formula:C21H15NO2
- Molecular Weight:313.3493
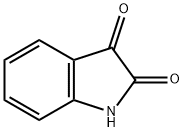
91-56-5
470 suppliers
$5.00/25g
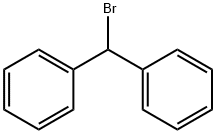
776-74-9
247 suppliers
$22.30/25g

94878-41-8
19 suppliers
$120.00/1g
Yield: 95%
Reaction Conditions:
Stage #1:indole-2,3-dione with sodium hydride in N,N-dimethyl-formamide at 0; for 1 h;
Stage #2:Bromodiphenylmethane in N,N-dimethyl-formamide at 20; for 4 h;
Steps:
20
Intermediate-20: 1 -Benzhydrylindoline-2,3-dione:To a stirred solution of isatin (20.0 g, 136 mmol) in dry DMF (500 ml) at 0 °C was added NaH (4.1 g, 170 mmol) step wise. The solution was stirred for lh at 0 °C. The benhydrin bromide (50 g, 200 mmol) was added lot wise. The color of the reaction mixture changes from brownish black to tar black. The course of reaction was monitored with TLC. After addition the reaction was stirred at room temperature for 4 h. The reaction mixture was quenched with addition of ice water, the phases were separated. The reaction mixture was extracted with EtOAc (2 x 250 ml). The combined organic extracts were washed with water and brine, dried over anhydrous sodium sulphate, filtered and concentrated in vacuo. The residue was purified by column chromatography, with an isocratic elution of 40% ethyl acetate in petroleum ether to afford the title compound (40.0 g, 95%) as a bright orange solid. NMR (400 MHz, CDC13): δ 7.12 (d, J = 8 Hz, 1H), 6.54 (dd, J= 8 Hz, 4 Hz, 1H), 6.41 (d, J= 4 Hz, 1H), 3.85 (s, 3H), 3.45 (s, 2H), 3.18 (s, 3H); MS (ES+) m/z 177.9 (M+l).
References:
LUPIN LIMITED;PADIYA, Kamlesh, Jyotindra;NAIR, Prathap, S.;PAL, Ravindra, Ramsurat;CHAURE, Ganesh, Shankar;GUDADE, Ganesh, Bhausaheb;PARKALE, Santosh, Sadashiv;MANOJKUMAR, Vishwanath, Lohar;SWAPNIL, Ramesh, Bajare;SMITA, Aditya, Bhoskar;SACHIN, Dilip, Survase;PALLE, Venkata, P.;KAMBOJ, Rajender, Kumar WO2012/49555, 2012, A1 Location in patent:Page/Page column 57