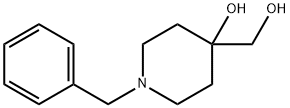
1-Benzyl-4-hydroxyMethylpiperidin-4-ol synthesis
- Product Name:1-Benzyl-4-hydroxyMethylpiperidin-4-ol
- CAS Number:92197-36-9
- Molecular formula:C13H19NO2
- Molecular Weight:221.3
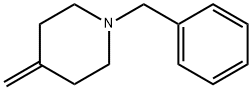
109105-86-4

92197-36-9
A3a) Synthesis of 1-benzyl-4-(hydroxymethyl)piperidin-4-ol: 200 g of AD-Mix-Alpha (Aldrich, Art. No. 39,275-8) was dissolved in a mixed solvent of 500 mL of water and 300 mL of tert-butanol, and stirred for 20 hours. The reaction mixture was cooled to 0 °C and 13.7 g (144 mmol) of methanesulfonamide and 27.0 g (144 mmol) of 1-benzyl-4-methylene piperidine were added sequentially. After removing the cooling bath, stirring was continued at room temperature for 22 hours. To the reaction mixture was added 59 g of Na2SO3 and stirred at room temperature for 1 hour. Subsequently, 2 L of EtOAc and 500 mL of saturated NaHCO3 solution were added and the organic phase was separated and dried over Na2SO4. After removing the desiccant and solvent, the residue was dissolved in 150 mL EtOAc and filtered through an alumina (Alox) column. The filtrate was discarded and the product was eluted from the Alox column with 1 L of MeOH. After evaporation of the solvent, the resulting product was used for subsequent reactions without further purification. Yield: 26.0 g (82% yield).ESI-MS: (M + H)+ = 222.HPLC retention time (Method B): 1.4 min.

109105-86-4
11 suppliers
inquiry

92197-36-9
22 suppliers
inquiry
Yield:92197-36-9 82%
Reaction Conditions:
Stage #1: 1-benzyl-4-methylene piperidenewith potassium osmate(VI);methanesulfonamide;water;potassium carbonate;potassium hexacyanoferrate(III);hydroquinidein 1,4-phthalazinediyl diether in tert-butyl alcohol at 0 - 20; for 22 h;
Stage #2: with sodium sulfite in tert-butyl alcohol at 20; for 1 h;
Steps:
A3a
A3a) 1-benzyl-4-hydroxymethyl-piperidin-4-ol A solution of 200 g AD-Mix-Alpha (Messrs Aldrich, Item no. 39,275-8) in 500 mL water and 300 mL tert-butanol was stirred for 20 min at RT, cooled to 0° C., combined with 13.7 g (144 mmol) methanesulphonic acid amide and 27.0 g (144 mmol) of 1-benzyl-4-methylene-piperidine and, after removal of the cooling bath, stirred for 22 h at RT. 59 g Na2SO3 were added to the reaction mixture and it was stirred for 1 h at RT. 2 L EtOAc and 500 mL saturated NaHCO3 solution were added, the organic phase was separated off and dried over Na2SO4 . After the desiccant and solvent had been eliminated the residue was dissolved in 150 mL EtOAc and filtered through Alox. The filtrate was discarded and the product was eluted from the Alox with 1 L MeOH. After the solvent had been eliminated the product was reacted further without purification. Yield: 26.0 g (82% of theory) ESI-MS: (M+H)+=222 retention time (HPLC): 1.4 min (method B)
References:
US2005/256099,2005,A1 Location in patent:Page/Page column 159
![6-benzyl-1-oxa-6-azaspiro[2,5]octane](/CAS/GIF/19867-34-6.gif)
19867-34-6
43 suppliers
inquiry

92197-36-9
22 suppliers
inquiry
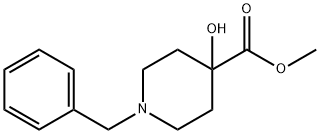
60437-30-1
47 suppliers
$95.00/250mg

92197-36-9
22 suppliers
inquiry
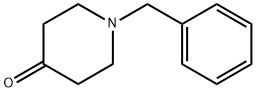
3612-20-2
449 suppliers
$6.00/10g

92197-36-9
22 suppliers
inquiry