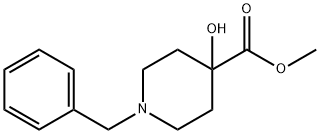
Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate synthesis
- Product Name:Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate
- CAS Number:60437-30-1
- Molecular formula:C14H19NO3
- Molecular Weight:249.31

67-56-1
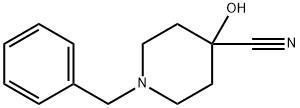
6094-60-6

60437-30-1
Example 1A Synthesis of methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate: 10.52 g (48.64 mmol) 1-benzyl-4-hydroxypiperidine-4-carbonitrile was dissolved in 60 mL of concentrated hydrochloric acid. The reaction mixture was stirred at 90°C for 1 hour. Upon completion of the reaction, the reaction solution was concentrated using a rotary evaporator and dried under high vacuum. The resulting residue was dissolved in a mixture of 150 mL of methanol and 6 mL of concentrated hydrochloric acid. Sulfuric acid was added to the solution and the mixture was subsequently stirred at 50°C for 1 hour. The reaction mixture was cooled, diluted with ethyl acetate and alkalized with saturated sodium carbonate solution. The organic phase was washed with sodium chloride solution, dried over sodium sulfate and finally concentrated by rotary evaporator. 10.8 g (43.6 mmol, 90% yield) of the target product was obtained. The product was analyzed by LC-MS (Method 4): retention time Rt = 2.08 min. Mass spectrum (ESI positive ion mode): m/z = 250 ([M + H]+). 1H NMR (400 MHz, DMSO-d6) data: δ = 7.35-7.2 (m, 5H), 5.28 (s, 1H), 3.63 (s, 3H), 3.45 (s, 2H), 2.53-2.4 (m, 2H, partially masked by DMSO), 2.38-2.2 (m, 2H, partially masked by DMSO), 2.38-2.2 (m, 2H). 2.38-2.2 (m, 2H), 1.9-1.78 (m, 2H), 1.59 (d, 2H).

67-56-1
790 suppliers
$7.29/5ml-f

6094-60-6
69 suppliers
$14.00/1g

60437-30-1
47 suppliers
$95.00/250mg
Yield: 90%
Reaction Conditions:
Stage #1:1-benzyl-4-hydroxypiperidine-4-carbonitrile with hydrogenchloride;water at 90; for 1 h;
Stage #2:methanol;sulfuric acid at 50; for 1 h;
Steps:
1A Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate
Example 1A Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate A solution of 10.52 g (48.64 mmol) of 1-benzyl-4-hydroxypiperidine-4-carbonitrile in 60 ml of conc. hydrochloric acid is stirred for one hour at 90° C. The reaction solution is concentrated on a rotary evaporator and dried under high vacuum. The residue obtained is taken up in 150 ml of methanol, 6 ml of conc. sulfuric acid are added and the mixture stirred for 1 hour at 50° C. After cooling the reaction mixture is diluted with ethyl acetate and rendered alkaline with a saturated sodium carbonate solution. The organic phase is washed with a sodium chloride solution, dried over sodium sulfate and concentrated on a rotary evaporator. 10.8 g (43.6 mmol, 90% th.) of product are obtained. LC-MS (method 4): Rt=2.08 min. MS (ESIpos): m/z=250 (M+H)+ 1H NMR (400 MHz, DMSO-d6): δ=7.35-7.2 (m, 5H), 5.28 (s, 1H), 3.63 (s, 3H), 3.45 (s, 2H), 2.53-2.4 (m, 2H, partly masked by DMSO), 2.38-2.2 (m, 2H), 1.9-1.78 (m, 2H), 1.59 (d, 2H).
References:
Location in patent:Page/Page column 16

6094-60-6
69 suppliers
$14.00/1g

60437-30-1
47 suppliers
$95.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f
59119-18-5
49 suppliers
$45.00/25mg

60437-30-1
47 suppliers
$95.00/250mg
3612-20-2
447 suppliers
$6.00/10g

60437-30-1
47 suppliers
$95.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f

3612-20-2
447 suppliers
$6.00/10g
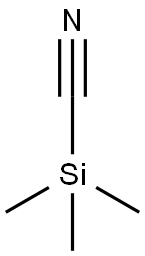
7677-24-9
395 suppliers
$19.00/5g

60437-30-1
47 suppliers
$95.00/250mg