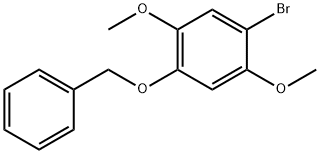
1-(BENZYLOXY)-4-BROMO-2,5-DIMETHOXYBENZENE synthesis
- Product Name:1-(BENZYLOXY)-4-BROMO-2,5-DIMETHOXYBENZENE
- CAS Number:524713-43-7
- Molecular formula:C15H15BrO3
- Molecular Weight:323.18

74-88-4
357 suppliers
$15.00/10g

524713-43-7
13 suppliers
inquiry
Yield:524713-43-7 88%
Reaction Conditions:
Stage #1: 5-benzyloxy-2-bromo-4-methoxyphenolwith sodium hydride in tetrahydrofuran at 0; for 0.333333 h;
Stage #2: methyl iodide in tetrahydrofuran at 20; for 18 h;
Steps:
2
5-Benzyloxy-2-bromo-4-methoxy-phenol 3 (2.76 g, 89.0 mmol) and NaH (0.89 g, 13.0 mmol, 60% dispersion in oil) were added to a flask and flushed with Ar. Dry THF (50 mL) was added and the suspension was stirred in an ice bath for 20 min. CH3I (1.7 mL, 27.0 mmol, filtered through basic alumina) was added and the mixture stirred at room temperature under Ar for 18 h. After cooling the reaction mixture in an ice bath, water was added slowly. The mixture was extracted with ethyl acetate, dried and concentrated to give yellow oil that solidified under vacuum. The oil was purified by silica gel chromatography using silica gel with (10% ethyl acetate/hexanes) to give 2.5 g (88%) of 4 as a white solid. 1H-NMR (400 MHz, CDCl3) dH 3.75 (3H, s, OCH3), 3.84 (3H, s, OCH3), 5.15 (2H, s, CH2Ph), 6.57 (11H, s, ArH), 7.07 (1H, s, ArH), 7.32-7.42 (5H, m, CH2Ph).
References:
US2006/257337,2006,A1 Location in patent:Page/Page column 17
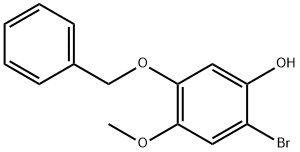
524713-42-6
12 suppliers
$350.95/1g

74-88-4
357 suppliers
$15.00/10g

524713-43-7
13 suppliers
inquiry
2973-59-3
250 suppliers
$6.00/1g

524713-43-7
13 suppliers
inquiry
6451-86-1
99 suppliers
$29.00/250mg

524713-43-7
13 suppliers
inquiry