
1-BOC-3-(4-CHLOROPHENYL)-3-HYDROXYPYRROLIDINE synthesis
- Product Name:1-BOC-3-(4-CHLOROPHENYL)-3-HYDROXYPYRROLIDINE
- CAS Number:324785-29-7
- Molecular formula:C15H20ClNO3
- Molecular Weight:297.78
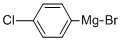
873-77-8
150 suppliers
$61.21/100ml
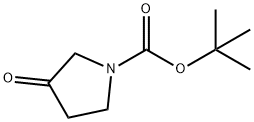
101385-93-7
482 suppliers
$5.00/1g

324785-29-7
14 suppliers
inquiry
Yield:324785-29-7 58%
Reaction Conditions:
Stage #1: (4-chlorphenyl)magnesium bromide;3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester in tetrahydrofuran;diethyl ether at 20;
Stage #2: with water;ammonium chloride in tetrahydrofuran;diethyl ether;
Steps:
81.A
EXAMPLE 814- (4-f 3 -(4-Chloro-phenyl)-pyrrolidin-3 -yl] -phenyl) - 1 H-pyrazole formate8 IA. 3 -(4-Chloro-phenyl)-3 -hydroxy-pyrrolidine- 1-carboxy lie acid tert-butyl ester; To a solution of 3 -oxo-pyrrolidine- 1-carboxy lie acid tert-butyl ester (1.Og, 5.40mmol) in anhydrous tetrahydrofuran (30ml) stirring at room temperature under nitrogen was added dropwise a solution of 4-chlorophenylmagnesium bromide (IM in ether, 27ml, 27mmol). After stirring thus overnight, the reaction was quenched by cautious addition of dilute aqueous ammonium chloride solution followed by addition of ethyl acetate. The mixture was filtered under suction then the aqueous was separated and extracted again with ethyl acetate. The organic liquors were combined, washed with saturated ammonium chloride then brine, dried (MgSO^ and concentrated in vacuo. The crude product was purified by silica Biotage column, eluting 20- 50% ethyl acetate/ petrol to furnish the title compound (935mg, 58%). LC/MS (PS-A2) R13.21 [M+H]+298
References:
WO2006/136830,2006,A1 Location in patent:Page/Page column 193
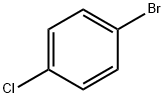
106-39-8
462 suppliers
$14.00/5g

101385-93-7
482 suppliers
$5.00/1g

324785-29-7
14 suppliers
inquiry

101385-93-7
482 suppliers
$5.00/1g

324785-29-7
14 suppliers
inquiry