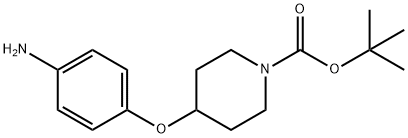
1-BOC-4-(4-AMINO-PHENOXY)-PIPERIDINE synthesis
- Product Name:1-BOC-4-(4-AMINO-PHENOXY)-PIPERIDINE
- CAS Number:138227-63-1
- Molecular formula:C16H24N2O3
- Molecular Weight:292.37
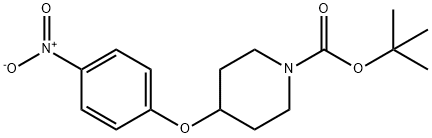
138227-62-0

138227-63-1
The general procedure for the synthesis of 1-Boc-4-(4-aminophenoxy)piperidine from tert-butyl 4-(4-nitrophenoxy)piperidine-1-carboxylate was as follows: 4-(1-tert-butoxycarbonylpiperidin-4-yloxy)nitrobenzene (11.9 g) obtained in Reference Example 105 was dissolved in methanol (100 mL) and palladium-carbon catalyst (1.9 g) was added. The reaction mixture was stirred at room temperature for 4 hours in a hydrogen atmosphere. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under reduced pressure. The resulting crude product was purified by silica gel column chromatography with the eluent being a solvent mixture of hexane and ethyl acetate in the ratio of 1:1, v/v. The final product was 1-Boc-4-(4-aminophenoxy)piperidine (10.7 g, 99% yield) as a light red solid.1H NMR (400 MHz, CDCl3) δ ppm: 1.46 (9H, s), 1.71 (2H, m). 1.87 (2H, m), 3.27 (2H, m), 3.71 (2H, m), 4.26 (1H, m), 6.63 (2H, d, J = 8.5 Hz), 6.76 (2H, d, J = 8.5 Hz).

138227-62-0
53 suppliers
$75.00/100mg

138227-63-1
100 suppliers
$45.00/50mg
Yield:138227-63-1 99%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol at 20; for 4 h;
Steps:
106 Reference example 106; 4-(1-t-Butoxycarbonylpiperidin-4-yloxy)aniline
To a solution of 4-(1-t-butoxycarbonylpiperidin-4-yloxy)nitrobenzene (11.9 g) obtained in reference example 105 in methanol (100 ml) was added palladium on carbon (1.9 g), and the resulting mixture was stirred at room temperature under a hydrogen atmosphere for 4 hours. At the end of this time, the reaction mixture was filtered, and the filtrate was evaporated in vacuo. The residue obtained was purified by chromatography on a silica gel column using a mixed solvent of hexane and ethyl acetate (1:1) as the eluent to afford the title compound (10.7 g, yield: 99 %) as a pale red solid. 1H NMR (400MHz, CDCl3) δ ppm : 1.46 (9H, s), 1.71 (2H, m), 1.87 (2H, m), 3.27 (2H, m), 3.71 (2H, m), 4.26 (1H, m), 6.63 (2H, d, J=8.5), 6.76 (2H, d, J=8.5).
References:
EP1375482,2004,A1 Location in patent:Page 159

141-78-6
683 suppliers
$22.37/250ml

138227-62-0
53 suppliers
$75.00/100mg

138227-63-1
100 suppliers
$45.00/50mg
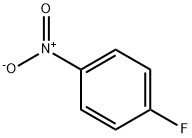
350-46-9
522 suppliers
$10.60/1gm:

138227-63-1
100 suppliers
$45.00/50mg
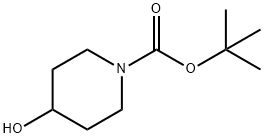
109384-19-2
420 suppliers
$5.00/1g

138227-63-1
100 suppliers
$45.00/50mg
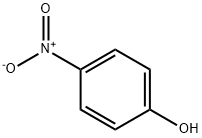
100-02-7
61 suppliers
$11.00/5G

138227-63-1
100 suppliers
$45.00/50mg