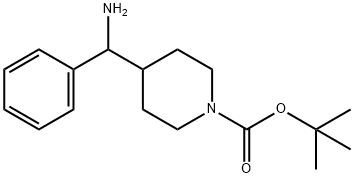
1-Boc-4-[amino(phenyl)methyl]piperidine synthesis
- Product Name:1-Boc-4-[amino(phenyl)methyl]piperidine
- CAS Number:612532-09-9
- Molecular formula:C17H26N2O2
- Molecular Weight:290.4

193217-39-9
72 suppliers
$75.00/100mg
![1-Boc-4-[amino(phenyl)methyl]piperidine](/CAS/20180629/GIF/612532-09-9.gif)
612532-09-9
13 suppliers
$212.00/250mg
Yield: 90%
Reaction Conditions:
Stage #1:1-(t-butoxycarbonyl)-4-(benzoyl)piperidine with ammonium acetate in methanol at 25; for 0.166667 h;
Stage #2: with methanol;sodium cyanoborohydride at 60; for 16 h;
Steps:
Synthesis of tert-butyl 4-(amino(phenyl)mefhyl)piperidine-l-carboxylate½r/-butyl 4-benzoylpiperidine-l-carboxylate (484 mg, 1.67 mmol) was dissolved in MeOH (15mL) and then NH4OAc (1.54 g, 20 mmol) was added. Reaction was stirred for 10 minutes at 25 °C. NaCNBH3 (420 mg, 6.68 mmol) was then added and the reaction was heated to 60 °C and stirred at that temperature for 16 hours. The solvent was removed under reduced pressure and the reaction mixture was suspended in 0.5 M NaOH (75 mL). Extracted with EtOAc (3 x 20 mL) and then dried with MgS04 and then under reduced pressure to give the title compound: clear oil (435 mg, 90%) NMR (400 MHz, CHLOROFORM-d) δ ppm 7.33 (m, .7=7.30 Hz, 2 H), 7.28 (d, 7=4.77 Hz, 3 H), 4.19 (br. s, 1 H), 4.02 (br. s, 1 H), 3.62 (d, 7=7.78 Hz, 1 H), 2.62 - 2.74 (m, 1 H), 2.48 - 2.61 (m, 1 H), 1.93 (d, 7=12.55 Hz, 1 H), 1.50 - 1.69 (m, 4 H), 1.44 (s, 9 H), 1.25 - 1.33 (m, 1 H), 1.00 - 1.14 (m, 1 H)
References:
UNIVERSITY HEALTH NETWORK;LAUFER, Radoslaw;PAULS, Heinz W.;FEHER, Miklos;NG, Grace;LIU, Yong;EDWARDS, Louise G.;PATEL, Narendra Kumar B.;PAN, Guohua;MAK, Tak W. WO2011/123937, 2011, A1 Location in patent:Page/Page column 104
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
183 suppliers
$6.00/100mg
![1-Boc-4-[amino(phenyl)methyl]piperidine](/CAS/20180629/GIF/612532-09-9.gif)
612532-09-9
13 suppliers
$212.00/250mg
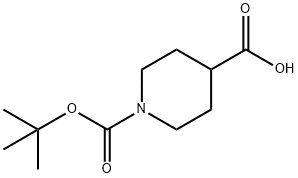
84358-13-4
379 suppliers
$5.00/5g
![1-Boc-4-[amino(phenyl)methyl]piperidine](/CAS/20180629/GIF/612532-09-9.gif)
612532-09-9
13 suppliers
$212.00/250mg

24424-99-5
862 suppliers
$13.50/25G
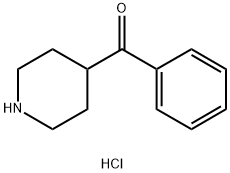
25519-80-6
94 suppliers
$22.32/Price $/250mgs:
![1-Boc-4-[amino(phenyl)methyl]piperidine](/CAS/20180629/GIF/612532-09-9.gif)
612532-09-9
13 suppliers
$212.00/250mg