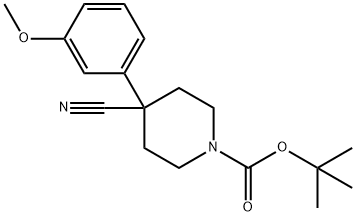
1-BOC-4-CYANO-4-(3-METHOXYPHENYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYANO-4-(3-METHOXYPHENYL)-PIPERIDINE
- CAS Number:553631-35-9
- Molecular formula:C18H24N2O3
- Molecular Weight:316.39
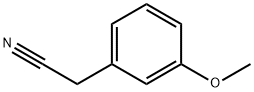
19924-43-7
262 suppliers
$9.00/10g
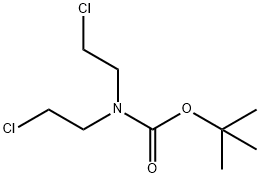
118753-70-1
178 suppliers
$6.00/1g

553631-35-9
17 suppliers
inquiry
Yield:553631-35-9 88.3 %
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 70;
Steps:
50.1 Step 1
Tert-butyl 4-cyano-4-(3-methoxyphenyl)piperidine-1-carboxylate 2-(3-methoxyphenyl)acetonitrile 50a (2 g,13.59 mmol) and tert-butyl bis(2-chloroethyl)carbamate (3.62 g, 14.95 mmol) were dissolved in N,N-dimethylformamide(12 mL), added with 60% sodium hydride (2.17 g, 54.36 mmol) in batches, stirred for 40 minutes, heated to 70°C, andreacted overnight. The reaction solution was cooled to room temperature, quenched with water (100 mL), and extractedwith ethyl acetate (100 mL33). Organic phases were combined, washed with a saturated sodium chloride solution (100mL), dried with anhydrous sodium sulfate and concentrated under reduced pressure. The obtained residue was purifiedby silica gel column chromatography (eluent: system A) to obtain tert-butyl 4-cyano-4-(3-methoxyphenyl)piperidine-1-carboxylate 50b (3.8 g) with a yield of 88.3%.MS m/z (ESI): 217.0 [M-99]
References:
EP4092019,2022,A1 Location in patent:Paragraph 0280-0281