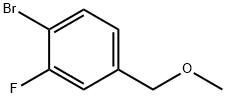
1-Bromo-2-fluoro-4-(methoxymethyl)benzene synthesis
- Product Name:1-Bromo-2-fluoro-4-(methoxymethyl)benzene
- CAS Number:162744-47-0
- Molecular formula:C8H8BrFO
- Molecular Weight:219.05
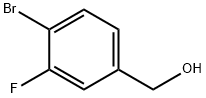
222978-01-0
117 suppliers
$11.00/250mg

74-88-4
357 suppliers
$15.00/10g

162744-47-0
52 suppliers
$45.00/10mg
Yield:162744-47-0 4.8 g
Reaction Conditions:
Stage #1: 4-bromo-3-fluorobenzyl alcoholwith sodium hydride in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: methyl iodide in tetrahydrofuran at 0 - 20; for 3 h;
Steps:
206B 1-bromo-2-fluoro-4-(methoxymethyl)benzene
To a solution of (4-bromo-3-fluorophenyl)methanol (5.0 g, 24 mmol) in tetrahydrofuran (150 mL) at 0 °C was added sodium hydride (1.4 g, 34 mmol). The reaction was stirred at this temperature for 10 min, and then methyl iodide (4.1 g, 29 mmol) was added. The reaction was allowed to warm from 0 °C to 20 °C over 3 hours, then quenched with saturated aqueous ammonium chloride (50 mL) and extracted with ethyl acetate (3 χ 100 mL). The combined organic layers were washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified by silica gel chromatography [petroleum ether: ethyl acetate = 30: 1] to give compound B- 308 (4.8 g, 90% yield) as an orange oil. TLC [petroleum ether: ethyl acetate = 10: 1]: Rf=0.5; - NMR (CDC13, 400 MHz): δ 7.53 (t, J=8.0 Hz, 1H), 7.14 (d, J=9.2 Hz, 1H), 7.01 (d, J=8.0 Hz, 1H), 4.43 (s, 2H), 3.42 (s, 3H).
References:
WO2016/100184,2016,A1 Location in patent:Paragraph 00677-00678

67-56-1
790 suppliers
$9.00/25ml

1240286-88-7
5 suppliers
inquiry

162744-47-0
52 suppliers
$45.00/10mg

1240286-88-7
5 suppliers
inquiry

162744-47-0
52 suppliers
$45.00/10mg
849758-12-9
124 suppliers
$7.00/250mg

162744-47-0
52 suppliers
$45.00/10mg

222978-01-0
117 suppliers
$11.00/250mg

162744-47-0
52 suppliers
$45.00/10mg