1-bromo-4-(propylthio)Benzene synthesis
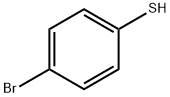
- Product Name:1-bromo-4-(propylthio)Benzene
- CAS Number:76542-19-3
- Molecular formula:C9H11BrS
- Molecular Weight:231.15
Yield:76542-19-3 100%
Reaction Conditions:
Stage #1: para-bromobenzenethiolwith sodium hydride in DMF (N,N-dimethyl-formamide) at 0;
Stage #2: propyl bromide in DMF (N,N-dimethyl-formamide) at 20;
Steps:
26
Dissolve 4-bromo-benzenethiol (2.0 g, 10.6 mmol) in dry dimethylformamide (50 mL) and cool to 0°C under nitrogen. Add sodium hydride (305 mg, 12. 7 mmol) in portions. After the vigorous gas evolution stops, add 1-bromo-propane (1.4 mL, 15.9 mmol) and stir the reaction mixture overnight at room temperature. Slowly pour the reaction into water (400 mL) and extract with diethyl ether (2 x 150 mL). Wash the combined organic layers with water (100 mL). Dry the organic layer with sodium sulfate, filter and concentrate in vacuo to yield 2.7 g of 1-bromo-4-propylsulfanyl-benzene (quantitative yield). Dissolve 1-bromo-4-propylsulfanyl-benzene (2.7g, 11.6 mmol) in dry tetrahydrofuran (100 mL) and cool the solution to-78°C. Add 2.5 M butyllithium in hexanes (5.1 mL, 12.8 mmol) dropwise and allow the reaction mixture to warm to-4. 0°C. Stir for 30 minutes and cool the reaction to-78°C. Add triisopropyl borate (8.0 mL, 34. 8 mmol) and allow the reaction mixture to slowly warm to room temperature. Add 10% aqueous potassium hydroxide (100 mL, 179 mmol) and stir overnight. Slowly pour the reaction mixture into a mixture of concentrated HCl and ice. Extract the aqueous solution with dichloromethane, dry with sodium sulfate and concentrate in vacuo to yield 1. 9 g of the title compound (86%).
References:
WO2004/9086,2004,A1 Location in patent:Page/Page column 74-75
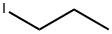
107-08-4
260 suppliers
$14.00/5g

76542-19-3
25 suppliers
inquiry
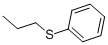
874-79-3
17 suppliers
$180.00/5g

76542-19-3
25 suppliers
inquiry