
1-Bromopropane synthesis
- Product Name:1-Bromopropane
- CAS Number:106-94-5
- Molecular formula:C3H7Br
- Molecular Weight:122.99
n-C3H7OH + HBr => n-C3H7Br + H2O
The prefix n (or normal) refers to a “straight chain” hydrocarbon component as opposed to a branched hydrocarbon component, such as the isopropyl group in isopropyl alcohol (i-propyl alcohol or i-propanol) where the hydroxyl (−OH) group is attached to the middle carbon.
Yield:106-94-5 97.8% ,75-26-3 1.57%
Reaction Conditions:
with hydrogen bromide;oxygen at 20 - 23; under 1551.49 - 1603.2 Torr; for 4.5 h;
Steps:
1
A laboratory-scale backpressure reactor was constructed using a heavy- walled, 60 psi (413.7 kPa) rated 500 rnL flask with threaded Teflon polymer coated connections to a 60 psi (413.7 kPa) rated condenser (50C coolant) and adapters with the vent gas connected to a back pressure regulator. An initial liquid volume of 178.70 g , (1.124 mol) NPB was added into the reactor. Hydrogen bromide gas was fed super- surface, through 0.5 inch (1.27 cm) Teflon polymer coated tubing increasing a rate of 0.67 g/min. for 3-4 minutes. Propene was fed through 0.25 inch (0.635 cm) PTFE tubing initially at approximately 205 mL/min though calibrated flow meters into a Fischer-Porter bottle. Oxygen-containing gas (air) (6 mL/min) was metered via computerized pump using No.14 Viton tubing into the Fischer-Porter bottle. The propene and air mixture was fed subsurface to the 500 mL flask during a 4.5 hour addition, during which HBr feed was maintained at approximately 0.67 gm/min. The hydrobromination was performed at 20- 23°C, 30-31 psi (206.8-213.7 kPa) with these pressures being maintained using a backpressure regulator. The liquid product was condensed as it was formed. The vent gas was scrubbed through water to remove HBr and the exit gas flow was measured intermittently with a gas (Bunte) buret. The calculated conversion to NPB, based on propene feed and hydrogen bromide exit gas amounts was 99.90 mole %. The crude EPO
References:
WO2006/113307,2006,A1 Location in patent:Page/Page column 18; 19
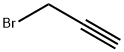
106-96-7
336 suppliers
$31.00/25g

106-94-5
583 suppliers
$10.00/5ml
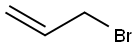
106-95-6
426 suppliers
$10.00/5g
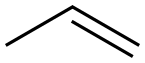
187737-37-7
1 suppliers
inquiry

106-94-5
583 suppliers
$10.00/5ml

74-98-6
129 suppliers
$40.00/1mL
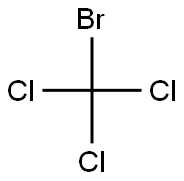
75-62-7
177 suppliers
$11.19/25G
201230-82-2
1 suppliers
inquiry
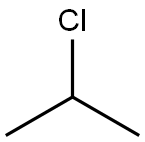
75-29-6
318 suppliers
$20.00/25mL

106-94-5
583 suppliers
$10.00/5ml
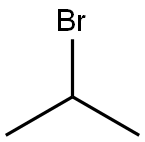
75-26-3
472 suppliers
$10.00/10g