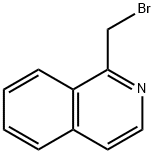
1-BROMOMETHYL-ISOQUINOLINE synthesis
- Product Name:1-BROMOMETHYL-ISOQUINOLINE
- CAS Number:74417-44-0
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
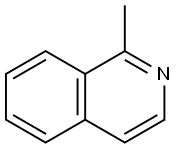
1721-93-3

74417-44-0
The general procedure for the synthesis of 1-(bromomethyl)isoquinoline from 1-methylisoquinoline is as follows: 1. 1-methylisoquinoline (0.99 g, 6.91 mmol), N-bromosuccinimide (NBS, 1.35 g, 1.1 eq.), azobisisobutyronitrile (AIBN, 10 mg, catalytic amount), and carbon tetrachloride (CCl4, 1 mL) were added to a reaction flask. 2. The reaction mixture was heated to reflux for 2 hours. 3. Upon completion of the reaction, the suspended solid particles were removed by filtration and the solids were washed with a small amount of CCl4. 4. The filtrate and washings were combined and concentrated under reduced pressure to remove the solvent. 5. The crude product was purified by column chromatography (eluent: 30% ethyl acetate/hexane) to afford 1-(bromomethyl)isoquinoline (270 mg, yield: 18%) as a violet solid. 6. The structure of the product was determined by 1H-1H-quinolysis. 6. The structure of the product was confirmed by 1H-NMR (500 MHz, CDCl3): δ 8.48 (d, 1H), 8.25 (d, 1H), 7.87 (d, 1H), 7.75-7.67 (m, 2H), 7.65 (d, 1H).

1721-93-3
127 suppliers
$19.00/1g

74417-44-0
58 suppliers
$38.00/100mg
Yield:74417-44-0 18%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane; for 2 h;Heating / reflux;
Steps:
17-1
Preparation 17-1); 1-Bromomethyl-isoquinolineTo 1-methylisoquinoline (0.99 g, 6.91 mmol), NBS (1.35 g, 1.1 eq) and AIBN(IO mg, catalytic amount) was added CCl4 (IS mL), which was then refluxed for 2 h. The suspended particles were removed by filtration, and washed with CCl4. The organic
References:
WO2008/16239,2008,A1 Location in patent:Page/Page column 57-58
27311-63-3
82 suppliers
$45.00/10mg

74417-44-0
58 suppliers
$38.00/100mg
4494-18-2
123 suppliers
$88.00/250mg

74417-44-0
58 suppliers
$38.00/100mg