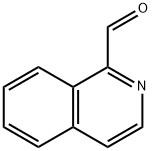
ISOQUINOLINE-1-CARBALDEHYDE synthesis
- Product Name:ISOQUINOLINE-1-CARBALDEHYDE
- CAS Number:4494-18-2
- Molecular formula:C10H7NO
- Molecular Weight:157.17
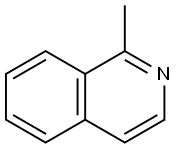
1721-93-3

4494-18-2
1. Synthesis of 1-isoquinolinecarboxaldehyde: 1-methylisoquinoline (300 mg, 2.10 mmol) was dissolved in 1,4-dioxane (20 mL). Selenium dioxide (323 mg, 2.91 mmol) was added to the solution, followed by heating and refluxing under nitrogen protection for 1.5 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was filtered through diatomaceous earth. The filtrate was distilled under reduced pressure to remove the solvent. The resulting residue was purified by silica gel column chromatography using n-hexane/ethyl acetate (5:1, v/v) as eluent, and the eluent grades containing the target product were collected, resulting in 1-isoquinolinecarboxaldehyde (252 mg, 77% yield) as a white solid. The structure of the product was confirmed by 1H-NMR (CDCl3) δ: 7.73-7.79 (m, 2H), 7.88-7.93 (m, 2H), 8.74-8.76 (m, 1H), 9.30-9.33 (m, 1H), 10.39 (s, 1H) and MS (ESI) m/z 158 (M++1).

1721-93-3
126 suppliers
$19.00/1g

4494-18-2
123 suppliers
$88.00/250mg
Yield: 77%
Reaction Conditions:
with selenium(IV) oxide in 1,4-dioxane;hexane;ethyl acetate
Steps:
102.1 (Step 1)
(Step 1) Synthesis of 1-isoquinolinecarbaldehyde In 1,4-dioxane (20 ml) was dissolved 1-methylisoquinoline (300 mg, 2.10 mmol). To the resulting solution was added selenium dioxide (323 mg, 2.91 mmol), followed by heating under reflux for 1.5 hours under a nitrogen gas stream. After cooling to room temperature, the reaction mixture was filtered through Celite. The filtrate was distilled under reduced pressure to remove the solvent. The residue was purified by chromatography on a silica gel column, whereby from n-hexane/ethyl acetate (5:1, v/v) eluate fractions, 1-isoquinolinecarbaldehyde (252 mg, 77%) was obtained as a white solid. 1H-NMR (CDCl3) δ: 7.73-7.79 (m, 2H), 7.88-7.93 (m, 2H), 8.74-8.76 (m, 1H), 9.30-9.33 (m, 1H), 10.39 (s, 1H). MS (ESI) m/z 158 (M++1).
References:
DAIICHI PHARMACEUTICAL CO., LTD. EP1346982, 2003, A1
119-65-3
428 suppliers
$5.00/25g
20461-86-3
34 suppliers
inquiry

4494-18-2
123 suppliers
$88.00/250mg
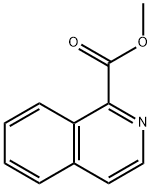
27104-72-9
66 suppliers
$75.00/250mg

4494-18-2
123 suppliers
$88.00/250mg
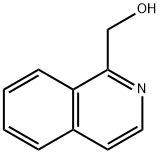
27311-63-3
82 suppliers
$45.00/10mg

4494-18-2
123 suppliers
$88.00/250mg

119-65-3
428 suppliers
$5.00/25g

4494-18-2
123 suppliers
$88.00/250mg