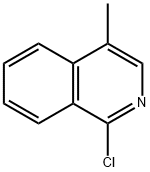
1-Chloro-4-Methylisoquinoline synthesis
- Product Name:1-Chloro-4-Methylisoquinoline
- CAS Number:24188-78-1
- Molecular formula:C10H8ClN
- Molecular Weight:177.63
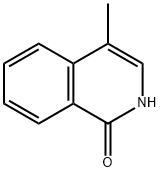
77077-83-9

24188-78-1
Step 2: Dissolve 4-methyl-2H-isoquinolin-1-one (4.8 g) in phosphorous trichloride (50 mL) and heat to reflux for 3 hours. Upon completion of the reaction, it was cooled to room temperature and concentrated under reduced pressure. The residue was treated with 5 N aqueous sodium hydroxide and subsequently extracted with dichloromethane. The organic layers were combined, washed with saturated brine and dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure and the resulting crude product was purified by Biotage fast chromatography, the eluent being a hexane solution of 5% ethyl acetate to give 4.8 g (90% yield) of the target product, 1-chloro-4-methylisoquinoline, as a white solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 2.59 (s, 3H), 7.68 (t, J = 7.70 Hz, 1H), 7.78 (m, 1H), 7.94 (d, J = 8.31 Hz, 1H), 8.11 (s, 1H), 8.35 (d, J = 8.31 Hz, 1H).

77077-83-9
13 suppliers
inquiry

24188-78-1
50 suppliers
$129.00/100mg
Yield:24188-78-1 90%
Reaction Conditions:
Stage #1: 4-methyl-1(2H)-isoquinolinonewith trichlorophosphate for 3 h;Heating / reflux;
Stage #2: with sodium hydroxide in water;
Steps:
2.B.86.2
Step 2: A solution of 4-methyl-2H-isoquinolin-1-one (4.8 g) in POCl3 (50 mL) was refluxed for 3 hours. After cooling and concentration, the residue was based with 5 N NaOH and extracted with CH2Cl2. The organic layer was washed with brine and dried over MgSO4. After filtration and concentration, purification by flash chromatography of Biotage with 5% ethyl acetate in hexanes gave 4.8 g (90%) of the desired product as a solid. 1H NMR (400 MHz, CDCl3) 8 ppm 2.59 (s, 3H), 7.68 (t, J=7.70 Hz, 1H), 7.78 (m, 1H), 7.94 (d, J-8.31 Hz, 1H), 8.11 (s, 1H), 8.35 (d, J=8.31 Hz, 1H).
References:
US2006/183694,2006,A1 Location in patent:Page/Page column 73-74
103261-29-6
0 suppliers
inquiry

24188-78-1
50 suppliers
$129.00/100mg
1196-39-0
57 suppliers
$245.00/100mg

24188-78-1
50 suppliers
$129.00/100mg
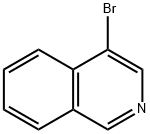
1532-97-4
360 suppliers
$14.00/10g

24188-78-1
50 suppliers
$129.00/100mg