
1-Chlorobutane synthesis
- Product Name:1-Chlorobutane
- CAS Number:109-69-3
- Molecular formula:C4H9Cl
- Molecular Weight:92.57

71-36-3
1090 suppliers
$14.00/25mL

109-69-3
447 suppliers
$20.00/100ml
Yield:109-69-3 96.9%
Reaction Conditions:
with hydrogenchloride in water at 115; for 2 h;Reagent/catalyst;Temperature;
Steps:
1-6 Example 5
Add 300ml of 2,3,5,6-tetramethyldioxane, 20ml of purified water and 200ml (2.19mol) of n-butanol to a three-necked flask with a capacity of 1000ml equipped with a stirrer and a thermometer. Stir and raise the temperature to 115 , 196.4g (5.38mol) of hydrogen chloride gas is introduced, and the reaction is distilled. As the reaction time increases, fractions are gradually distilled out. After 100ml of fractions are distilled out, use a peristaltic pump to feed the reaction flask at a rate of 3ml/min. Add n-butanol, add 800ml (8.74mol) of n-butanol in total, keep the hydrogen chloride gas at a 3g/min feed rate during the addition, and after completing the heat preservation distillation and reaction for 2h, Almost no fraction is distilled out further. The reaction is over. After the obtained fractions are layered, the upper organic layer is 1-chlorobutane. The final 1-chlorobutane is 980.6g (10.59mol), the molar yield is 96.9%, and the GC purity 99.89%.
References:
CN112707789,2021,A Location in patent:Paragraph 0039-0058
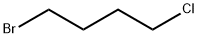
6940-78-9
365 suppliers
$5.00/10g

109-69-3
447 suppliers
$20.00/100ml

109-65-9
570 suppliers
$10.00/10g

109-69-3
447 suppliers
$20.00/100ml

71-36-3
1090 suppliers
$14.00/25mL

109-69-3
447 suppliers
$20.00/100ml
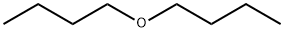
142-96-1
324 suppliers
$14.00/25mL

693-03-8
59 suppliers
$153.00/50mL
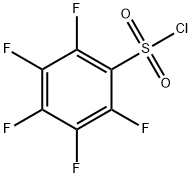
832-53-1
163 suppliers
$9.00/250mg

109-65-9
570 suppliers
$10.00/10g

109-69-3
447 suppliers
$20.00/100ml
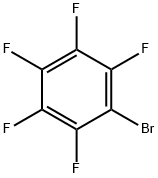
344-04-7
378 suppliers
$9.00/5g
363-72-4
294 suppliers
$10.00/1g