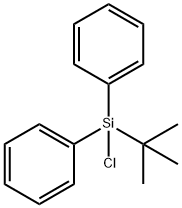
tert-Butylchlorodiphenylsilane synthesis
- Product Name:tert-Butylchlorodiphenylsilane
- CAS Number:58479-61-1
- Molecular formula:C16H19ClSi
- Molecular Weight:274.86
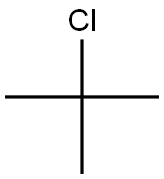
507-20-0
385 suppliers
$14.00/25mL
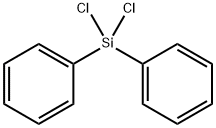
80-10-4
214 suppliers
$10.00/5g

58479-61-1
418 suppliers
$10.00/25g
Yield:58479-61-1 87%
Reaction Conditions:
Stage #1: tertiary butyl chloridewith magnesium in tetrahydrofuran at 60; for 2 h;
Stage #2: with lithium chloride;copper(I) bromide in tetrahydrofuran at 20; for 1 h;
Stage #3: diphenylsilyl dichloride in tetrahydrofuran at 50; for 6 h;Reagent/catalyst;Temperature;
Steps:
1-4; 2; 3; 5 Example 1
Into a flask equipped with a stirrer, a reflux condenser, a dropping funnel and a thermometer, 13.3 g (0.547 mol) of magnesium and 300 ml of tetrahydrofuran (hereinafter referred to as THF) were charged and heated to 60 ° C. To this reaction solution, 55.5 g (0.599 g of tert-butyl chloride) was added.Mol) was added over 1 hour and stirred at the same temperature for 1 hour to prepare tert-butylmagnesium chloride as a Grignard reagent.Next, this Grignard reagent is cooled to room temperature,Cuprous chloride (3.0 g, 0.030 mol) and lithium chloride (23.3 g, 0.550 mol) were added, and the mixture was stirred at room temperature for 1 hour.126.7 g (0.5004 mol) of diphenyldichlorosilane was added dropwise to the obtained reaction liquid over 1 hour, and then stirred at 70 ° C. for 5 hours. At this point, gas chromatographic analysis was performed to calculate the reaction rate. The results are shown in Table 1.
References:
JP2019/182793,2019,A Location in patent:Paragraph 0028-0031; 0033-0037

80-10-4
214 suppliers
$10.00/5g

58479-61-1
418 suppliers
$10.00/25g
6328-61-6
2 suppliers
inquiry

58479-61-1
418 suppliers
$10.00/25g
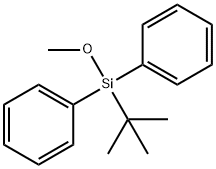
76358-47-9
27 suppliers
inquiry

58479-61-1
418 suppliers
$10.00/25g
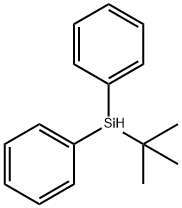
33729-92-9
76 suppliers
$45.00/50mg

58479-61-1
418 suppliers
$10.00/25g