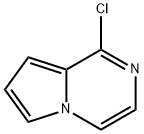
1-Chloro-1H-pyrrolo[1,2-a]pyrazine synthesis
- Product Name:1-Chloro-1H-pyrrolo[1,2-a]pyrazine
- CAS Number:136927-64-5
- Molecular formula:C7H5ClN2
- Molecular Weight:152.58
![Pyrrolo[1,2-a]pyrazin-1(2H)-one (9CI)](/CAS/GIF/136927-63-4.gif)
136927-63-4
![1-Chloro-1H-pyrrolo[1,2-a]pyrazine](/CAS/GIF/136927-64-5.gif)
136927-64-5
The general procedure for the synthesis of 1-chloropyrrolo[1,2-a]pyrazine from pyrrolo[1,2-a]pyrazin-1(2H)-one was as follows: pyrrolo[1,2-a]pyrazin-1(2H)-one (2.14 g, 16.0 mmol) was dissolved in phosphorus trichloride (20 mL), and the reaction was stirred for 16 h at room temperature. Upon completion of the reaction, the reaction mixture was quenched by slowly pouring it into ice water and neutralized with sodium bicarbonate solution. Subsequently, the aqueous phase was extracted several times with dichloromethane, and the combined organic phases were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the resulting residue was purified by silica gel column chromatography with 10% ethyl acetate/hexane as eluent to afford 1-chloropyrrolo[1,2-a]pyrazine (1.1 g, 45% yield). The structure of the product was analyzed by 1H NMR (500 MHz, CDCl3, δ: 7.62 (d, J = 4.5 Hz, 1H), 7.34 (dd, J = 2.5, 1.5 Hz, 1H), 7.12 (d, J = 4.5 Hz, 1H), 6.74-6.71 (m, 2H)), 13C NMR (125 MHz, CDCl3, δ. 154.4, 126.0, 125.6, 118.3, 117.4, 115.6, 105.2) and mass spectrometry (LRMS (ES): calculated value of C7H6ClN2 [M+1]+ 153, measured value of 153.2) confirmed.
![Pyrrolo[1,2-a]pyrazin-1(2H)-one (9CI)](/CAS/GIF/136927-63-4.gif)
136927-63-4
83 suppliers
$74.00/250mg

124-38-9
134 suppliers
$214.00/14L
![1-Chloro-1H-pyrrolo[1,2-a]pyrazine](/CAS/GIF/136927-64-5.gif)
136927-64-5
94 suppliers
$51.00/100mg
Yield:136927-64-5 71%
Reaction Conditions:
with sodium hydrogencarbonate;trichlorophosphate in acetone;
Steps:
20 (A compound of formula (BB))
PREPARATION 20 1-chloropyrrolo[1,2-a]pyrazine (A compound of formula (BB)) To pyrrolo[1,2-a]pyrazin-1(2H)-one (0.35 g, 2.61 mmol) was added phosphorous oxychloride (4.0 g, 26.1 mmol). The reaction mixture was agitated for approximately 16 hours. The reaction mixture was then made alkaline by the addition of sodium bicarbonate. The reaction mixture was then extracted with hexane, washed with a saturated sodium bicarbonate solution, dried over MgSO4 and evaporated. The product was purified by recrystallization in hexane and cooled with a mixture of dry ice and acetone to give the title compound, 1-chloropyrrolo[1,2-a]pyrazine (71%), m.p. 56°-57° C.
References:
US5041442,1991,A
![Pyrrolo[1,2-a]pyrazin-1(2H)-one (9CI)](/CAS/GIF/136927-63-4.gif)
136927-63-4
83 suppliers
$74.00/250mg
![1-Chloro-1H-pyrrolo[1,2-a]pyrazine](/CAS/GIF/136927-64-5.gif)
136927-64-5
94 suppliers
$51.00/100mg