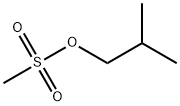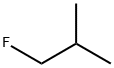
1-fluoro-2-methylpropane synthesis
- Product Name:1-fluoro-2-methylpropane
- CAS Number:359-00-2
- Molecular formula:C4H9F
- Molecular Weight:76.11
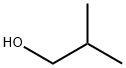
78-83-1
808 suppliers
$14.00/5mL

359-00-2
0 suppliers
inquiry
Yield:359-00-2 59.3%
Reaction Conditions:
with methyl fluorosulfonate;1,8-diazabicyclo[5.4.0]undec-7-ene at 60 - 100;Inert atmosphere;
Steps:
7 Example 5
Example 5
In a 300 ml volume glass reactor equipped with a stirring bar, a dropping funnel, a thermometer and a collection receiver, 57 parts of 2-butanol and 85.3 g of DBU were placed; then the contents were placed in a nitrogen atmosphere, and then the reactor was heated to 60° C.
When the temperature of the thermometer became constant, 57.2 g of the ethanesulfonyl fluoride produced in Production Example 2 was added dropwise to the reactor from the dropping funnel over approximately 1 hour. After the completion of the dropwise addition, the reaction was continued at 60° C. for thrther 5 hours, and then the reactor was increased in temperature to 100° C., and the reaction was continued for further 3 hours. Meanwhile, the distilled organic component was collected in a receiver immersed in a dry ice-ethanol bath. The organic substance collected in the receiver was analyzed with gas chromatography, and 23.0 g of isobutyl fluoride, the target product, was found to be obtained (yield with reference to methanesulfonyl fluoride: 59.3%).The structure of the target product was identified on the basis of the ‘H-NMR spectrum and the ‘9F-NMR spectrum.‘H-NMR (CDC13, TMS) ? ppm: 1.03 (t, 3Hx2), 1.97 (m, 1R), 4.41 (m, 2H), 4.45 (m, 2H).‘9F-NMR (CDC13, CFC13) ? ppm: -220 (m, F)
References:
US2018/118642,2018,A1 Location in patent:Paragraph 0195; 0196; 1097; 0198
![Lithium, tetrakis[μ3-(1-methylpropyl)]tetra- (9CI)](/CAS/20211123/GIF/90269-51-5.gif)
90269-51-5
0 suppliers
inquiry

359-00-2
0 suppliers
inquiry
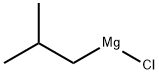
5674-02-2
84 suppliers
$88.50/100ml

359-00-2
0 suppliers
inquiry