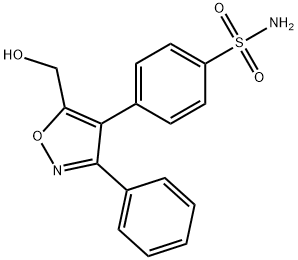
1-Hydroxy Valdecoxib synthesis
- Product Name:1-Hydroxy Valdecoxib
- CAS Number:181695-81-8
- Molecular formula:C16H14N2O4S
- Molecular Weight:330.36
![Benzenesulfonamide, 4-[5-(chloromethyl)-3-phenyl-4-isoxazolyl]-](/CAS/20210305/GIF/181696-34-4.gif)
181696-34-4
0 suppliers
inquiry

181695-81-8
15 suppliers
$1320.00/100mg
Yield:181695-81-8 69%
Reaction Conditions:
Stage #1: 4-(5-(chloromethyl)-3-phenylisoxazol-4-yl)benzenesulfonamidewith formic acid;triethylamine in acetonitrile at 80; for 5 h;
Stage #2: with lithium hydroxide monohydrate;sodium hydroxide in acetonitrile; pH=11; for 3 h;
Steps:
S-07.D Step D.
4-(5-(chloromethyl)-3-phenylisoxazol-4-yl)benzenesulfonamide(5g, 14.3 mmol), formic acid (2.9 g, 64.3mmol) and triethylamine (3.6 g, 35.7 mmol) were heated to reflux in acetonitrile (50 mL) for 5h. The pH of the solution was adjusted to 11 with NaOH (2.5 M aq., ~20 mL) and heated to reflux for 3h then cooled to room temperature. EtOAc (100 mL) and H2O (80 mL) were added, and the pH of the solution was adjusted to 2 by the addition of concentrated HCl. The layers were separated, and the organic layer was collected, washed with brine (100 mL), dried over Na2SO4, filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel chromatography, eluting with PE/EtOAc (60/40) to give 4-(5- (hydroxymethyl)-3-phenylisoxazol-4-yl)benzenesulfonamide (3.3 g, yield: 69 %) as a light yellow solid.1H NMR (400 MHz, DMSO) δ 7.88 - 7.80 (m, 2H), 7.51 - 7.39 (m, 7H), 7.40 - 7.34 (m, 2H), 5.78 (t, J = 5.8 Hz, 1H), 4.56 (d, J = 5.5 Hz, 2H). Mass Spectrum (ESI) m/z = 331 (M+1).
References:
WO2022/76741,2022,A1 Location in patent:Paragraph 0190
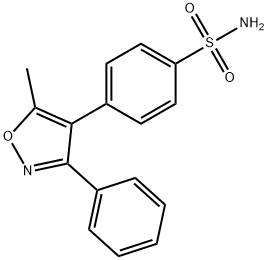
181695-72-7
345 suppliers
$5.00/100mg

181695-81-8
15 suppliers
$1320.00/100mg
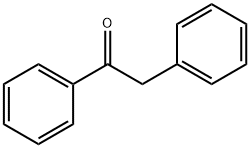
451-40-1
377 suppliers
$13.43/1gm:

181695-81-8
15 suppliers
$1320.00/100mg
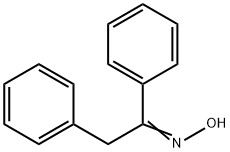
952-06-7
105 suppliers
$67.00/500mg

181695-81-8
15 suppliers
$1320.00/100mg
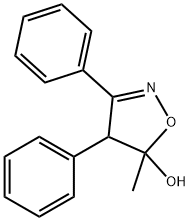
181696-73-1
109 suppliers
$120.50/50mg

181695-81-8
15 suppliers
$1320.00/100mg