
1-Iodododecane synthesis
- Product Name:1-Iodododecane
- CAS Number:4292-19-7
- Molecular formula:C12H25I
- Molecular Weight:296.23

112-53-8
482 suppliers
$10.00/5g

4292-19-7
161 suppliers
$18.00/1g
Yield:4292-19-7 99%
Reaction Conditions:
with 1H-imidazole;iodine;triphenylphosphine in dichloromethane at 20; for 1.5 h;Inert atmosphere;
Steps:
1.A
Dodecan-1-ol (10.0 g, 53.7 mmol, 1.00 eq) was added to a 1 L flask with a stir bar. Diluted with DCM (268 mL), and the resulting solution was stirred vigorously at room temperature. After addition of imidazole (4.75 g, 69.8 mmol, 1.30 eq), triphenylphosphine (18.3 g, 69.8 mmol, 1.30 eq), and iodine (17.7 g, 69.8 mmol, 1.30 eq) in succession, the resulting reaction mixture was stirred vigorously at room temperature under argon (Ar). Reaction progress was monitored by TLC. After 1.5 hrs, TLC indicated conversion of starting material to one major spot. The reaction mixture was quenched with 150 mL saturated aqueous sodium thiosulfate. The resulting organic layer was diluted with 200 mL hexanes, the small amount of precipitate was filtered, and the mother liquor was evaporated under reduced pressure to yield white solid. After addition of 400 mL hexanes, the resulting slurry was stirred vigorously overnight under Ar. In the next morning, the slurry was filtered, and the mother liquor was evaporated under reduced pressure to yield a yellow oil. The crude material was purified via silica plug eluting with 100% hexanes to yield a clear oil with a small amount of yellow solid remaining. This crude material was taken up in 100% pentane, filtered, and the mother liquor was evaporated under reduced pressure to yield a clear oil, which corresponded to the product 1-iodododecane (15.7 g, 53.0 mmol, 99% yield).
References:
EMORY UNIVERSITY WO2021/35214, 2021, A1 Location in patent:Page/Page column 131; 132
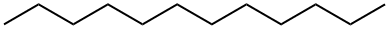
112-40-3
390 suppliers
$10.00/10g

4292-19-7
161 suppliers
$18.00/1g
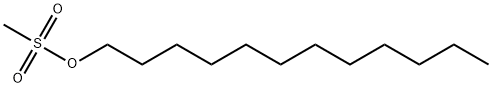
51323-71-8
11 suppliers
$144.00/100mg

4292-19-7
161 suppliers
$18.00/1g
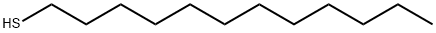
112-55-0
343 suppliers
$14.00/5mL

4292-19-7
161 suppliers
$18.00/1g

143-15-7
447 suppliers
$5.00/25g

4292-19-7
161 suppliers
$18.00/1g