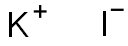
Potassium iodide synthesis
- Product Name:Potassium iodide
- CAS Number:7681-11-0
- Molecular formula:KI
- Molecular Weight:166
I2 + 6KOH → 5KI + KIO3 + 3H2O
Most potassium iodate, KIO3 , is separated from the product mixture by crystallization and filtration. Remaining iodates are removed by evaporation of the solution and other processes, such as carbon reduction or thermal decompostion at 600oC to iodide:
2KIO3 → 2KI + 3O2
Another method of preparation that does not involve the formation of iodate is by treating iron turnings with iodine solution. The product, ferrosoferric iodide, Fe3I8?16H2O, is boiled with 15 wt% potassium carbonate solution:
Fe3I8.16H2O + 4K2CO3 → 8 KI + 4CO2 + Fe3O4 + 16H2O
A similar method is used to prepare potassium bromide, discussed earlier (see Potassium Bromide.)
Potassium iodide can be prepared by reacting hydriodic acid with potassium bicarbonate:
HI + KHCO3 → KI + CO2 + H2O
It is purified by melting in dry hydrogen.
Potassium iodide also may be obtained by various electrolytic processes.
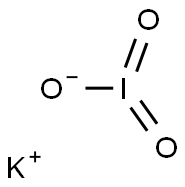
7758-05-6
428 suppliers
$44.00/100g
7681-11-0
1267 suppliers
$10.00/5g
Yield:-
Reaction Conditions:
platinum in not given;reduction;
References:
Gall, H.;Manchot, W. [Chemische Berichte,1925,vol. 58,p. 484]

7758-05-6
428 suppliers
$44.00/100g
10034-93-2
467 suppliers
$13.00/25g

7553-56-2
644 suppliers
$19.00/25g
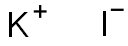
7681-11-0
1267 suppliers
$10.00/5g

7553-56-2
644 suppliers
$19.00/25g
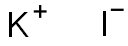
7681-11-0
1267 suppliers
$10.00/5g
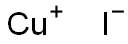
7681-65-4
577 suppliers
$6.00/5g
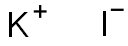
7681-11-0
1267 suppliers
$10.00/5g
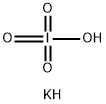
7790-21-8
230 suppliers
$11.00/5g
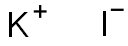
7681-11-0
1267 suppliers
$10.00/5g