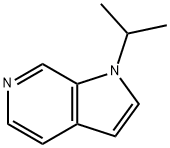
1-isopropyl-1H-pyrrolo[2,3-c]pyridine synthesis
- Product Name:1-isopropyl-1H-pyrrolo[2,3-c]pyridine
- CAS Number:1221153-83-8
- Molecular formula:C10H12N2
- Molecular Weight:160.22
271-29-4
272 suppliers
$9.00/250mg
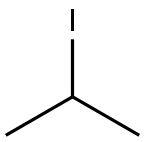
75-30-9
278 suppliers
$15.00/1g
![1-isopropyl-1H-pyrrolo[2,3-c]pyridine](/CAS/20180629/GIF/1221153-83-8.gif)
1221153-83-8
13 suppliers
$427.75/1g
Yield:-
Reaction Conditions:
Stage #1: 6-azaindolewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.75 h;Inert atmosphere;
Stage #2: 2-iodo-propane in N,N-dimethyl-formamide at 0 - 20;
Steps:
G
To a 40-mL scintillation vial fitted with a magnetic stir bar was added 6-azaindole G-I (0.30 g, 2.54 mmol, 1 eq.) and 5 mL anhydrous DMF. Nitrogen was bubbled thoroughly through the solution for 10 minutes. The solution was then cooled to 0 0C followed by the addition of sodium hydride (0.117 g of a 60% dispersion, 2.92 mmol, 1.1 eq.) and the mixture was allowed to stir for 45 minutes. 2-iodopropane (0.240 mL, 2.41 mmol, 0.95 eq.) was then added, and the reaction mixture was allowed to warm to room temperature. After three hours, the crude reaction mixture was concentrated in vacuo onto Celite, and purification by silica gel chromatography (gradient 0 to 10% MeOH in DCM over 12 min) afforded l-isopropyl-6-azaindole (G-2) as an off-white powder. LCMS m/e 161 (M+Η); 1H NMR (400 MHz, Chloroform-^/) δ ppm 1.64 (s, 3 H), 1.65 (s, 3 H), 4.72 - 4.93 (m, 1 H), 6.98 (d, J=5.3 Hz, 1 H), 8.07 (s, 1 H), 8.17 (dd, J=5.6, 0.7 Hz, 1 H), 8.28 (d, J=5.3 Hz, 1 H), 8.38 (d, J=5.5 Hz, 1 H), 8.87 (s, 1 H).
References:
WO2010/42337,2010,A1 Location in patent:Page/Page column 46; 47