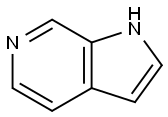
6-Azaindole synthesis
- Product Name:6-Azaindole
- CAS Number:271-29-4
- Molecular formula:C7H6N2
- Molecular Weight:118.14
![7-CHLORO-1H-PYRROLO[2,3-C]PYRIDINE](/CAS/GIF/357263-41-3.gif)
357263-41-3

271-29-4
General procedure for the synthesis of 6-azaindole using 7-chloro-1H-pyrrolo[2,3-c]pyridine as starting material: a suspension of 7-chloro-1H-pyrrolo[2,3-c]pyridine (650 mg, 4.2 mmol) with 10% Pd/C (50 mg) in ethanol (25 mL) was subjected to a hydrogen atmosphere (balloon pressure) and the reaction was stirred at room temperature. Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad and the filter cake was washed with ethyl acetate (10 mL). The filtrates were combined, concentrated under reduced pressure and subsequently purified by fast column chromatography (eluent: dichloromethane/methanol/ammonia, 9:1:0.1, v/v) to afford 1H-pyrrolo[2,3-c]pyridine as a white solid (380 mg, 75% yield).
![7-CHLORO-1H-PYRROLO[2,3-C]PYRIDINE](/CAS/GIF/357263-41-3.gif)
357263-41-3
182 suppliers
$45.00/50mg

271-29-4
272 suppliers
$9.00/250mg
Yield:271-29-4 75%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20;
Steps:
19.1
A suspension of 7-chloro-1H-pyrrolo[2,3-c]pyridine (650 mg, 4.2 mmol) and Pd/C (10%, 50 mg) in EtOH (25 mL) was stirred at RT under H2 atmosphere (balloon pressure). The reaction mixture was filtered through a celite pad, and the filter cake was washed with EtOAc (10 mL). The filtrate was concentrated and purified via flash chromatography (DCM/MeOH/NH4OH, 9/1/0.1), affording 1H-Pyrrolo[2,3-c]pyridine as a white solid (380 mg, 75% yield).
References:
Roche Palo Alto LLC US2007/123535, 2007, A1 Location in patent:Page/Page column 43
![tert-butyl 2-hydroxy-2,3-dihydro-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/20180703/GIF/185139-01-9.gif)
185139-01-9
5 suppliers
inquiry

271-29-4
272 suppliers
$9.00/250mg
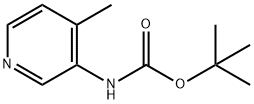
180253-66-1
39 suppliers
$45.00/50mg

271-29-4
272 suppliers
$9.00/250mg
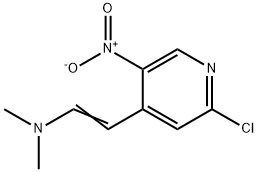
142078-36-2
26 suppliers
inquiry

271-29-4
272 suppliers
$9.00/250mg
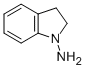
7633-56-9
10 suppliers
inquiry

271-29-4
272 suppliers
$9.00/250mg