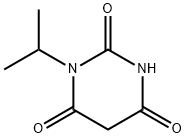
1-isopropylpyrimidine-2,4,6(1H,3H,5H)-trione synthesis
- Product Name:1-isopropylpyrimidine-2,4,6(1H,3H,5H)-trione
- CAS Number:69998-14-7
- Molecular formula:C7H10N2O3
- Molecular Weight:170.17
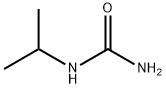
691-60-1
104 suppliers
$105.00/250mg

105-53-3
791 suppliers
$5.00/25g

69998-14-7
53 suppliers
inquiry
Yield: 79%
Reaction Conditions:
with sodium ethanolate in ethanol at 75 - 80; for 20.7 h;Large scale;
Steps:
13.1 Compound 1.2. Synthesis of 1-Isopropylpyrimidine-2,4,6(1H,3H,5H)-trione (Ethanol)
1-Isopropylurea (4.983 kg, 48.79 mol; compound 1.1), absolute ethanol (15.8 kg), diethyl malonate, (8.701 kg, 54.32 mol, 1.1 equiv), and sodium ethoxide (21 wt% in ethanol), (20.7 kg, 63.9 mol, 1.3 equiv) were added to a 100 L reactor and heated to reflux (75-80 °C) with stirring (145 rpm) for 20.7 hours. An IPC LC/MS limit test indicated ≤ 10% 1- isopropylurea. The mixture was cooled to 24 °C. A solution of 2N HCl was prepared by mixing potable water (30.0 kg) and concentrated HCl (6.3 kg). The 2N HCl solution was added to the reaction mixture over 25 min (23-25 °C temperature range), adjusting the pH to 3. The slurry was then concentrated by vacuum distillation to about 27 L (5.5 L/kg), while maintaining a pot temperature below 50 °C. An IPC GC headspace limit test indicated ≤ 10% ethanol. The slurry was cooled to 9 °C and mixed for 15.5 h at 5-10 °C. The solids were isolated by filtration, washed with potable water (30.0 kg) and vacuum-dried at 40-45 °C for 39 h. The dried product afforded 6.579 kg (79%) of 1-isopropylpyrimidine-2,4,6(1H,3H,5H)-trione (compound 1.2) as a light yellow solid in 99.22% purity (a/a).
References:
MYOKARDIA, INC. WO2021/92598, 2021, A1 Location in patent:Paragraph 567

691-60-1
104 suppliers
$105.00/250mg
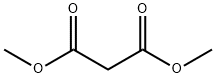
108-59-8
648 suppliers
$5.00/25g

69998-14-7
53 suppliers
inquiry