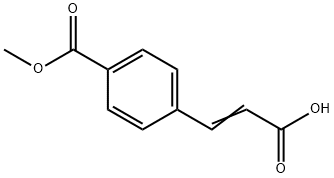
1-methyl 4-(2-carboxyvinyl)benzoate synthesis
- Product Name:1-methyl 4-(2-carboxyvinyl)benzoate
- CAS Number:19473-96-2
- Molecular formula:C11H10O4
- Molecular Weight:206.19
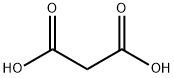
141-82-2
777 suppliers
$5.00/25g
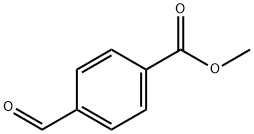
1571-08-0
343 suppliers
$10.00/5g

19473-96-2
25 suppliers
$114.00/1g
Yield:19473-96-2 100%
Reaction Conditions:
with piperidine;pyridine at 120; for 12 h;
Steps:
1 Example 1: A preparation method of Compound 1, the process is as follows:
38. 1g of methyl p-formylbenzoate and 31.2g of malonic acid were added to the reactor, and then 200 mL of pyridine and 11.6 mL of piperidine were added. The reaction mixture was heated to 120 ° C and refluxed for 12 h. After the reaction, it was cooled to At room temperature, an acidic ice water solution was prepared with concentrated hydrochloric acid, and the reaction liquid was slowly poured into a large beaker containing ice water, stirred while being added, and adjusted with appropriate hydrochloric acid until the final ice water solution was acidic. At this time, a large amount of white solid was precipitated, and after suction filtration, the solid was washed with water three times, and the filter cake was dried to obtain a white solid compound (147.8 g, yield: 100%; TLC ( petroleum ether: ethyl acetate = 2:1, Rf = 0.7),The GF254 was burned under the visible not only one point, but also the compound 1.
References:
CN109721604,2019,A Location in patent:Paragraph 0043; 0044
![Benzoic acid, 4-[3-(1,1-dimethylethoxy)-3-oxo-1-propen-1-yl]-, methyl ester](/CAS/20210111/GIF/188979-84-2.gif)
188979-84-2
1 suppliers
inquiry

19473-96-2
25 suppliers
$114.00/1g
73166-52-6
2 suppliers
inquiry

19473-96-2
25 suppliers
$114.00/1g
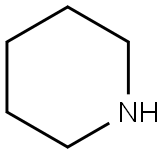
110-89-4
0 suppliers
$15.96/100ML

1571-08-0
343 suppliers
$10.00/5g

19473-96-2
25 suppliers
$114.00/1g
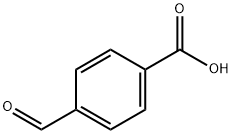
619-66-9
435 suppliers
$5.00/5g

19473-96-2
25 suppliers
$114.00/1g