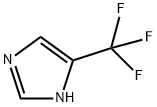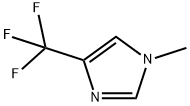
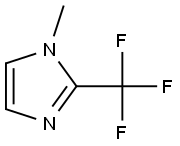
1-Methyl-4-(trifluoromethyl)-1H-imidazole synthesis
- Product Name:1-Methyl-4-(trifluoromethyl)-1H-imidazole
- CAS Number:81769-69-9
- Molecular formula:C5H5F3N2
- Molecular Weight:150.1
Yield:81769-69-9 83 %
Reaction Conditions:
Stage #1: 4-(trifluoromethyl)-1H-imidazolewith sodium hydride in tetrahydrofuran at 0;
Stage #2: methyl iodide in tetrahydrofuran at 20;
Steps:
28.1 Step 1: Synthesis of 1-methyl-4-(trifluoromethyl)-1H-imidazole (30B)
4-(Trifluoromethyl)-1H-imidazole (5.0g, 37mmol) was dissolved in anhydrous tetrahydrofuran (50mL), and sodium hydride (content 60%) (1.6g, 68mmol) was added thereto under ice-cooling conditions , continue to react at this temperature for 30min, then add iodomethane (5.2g, 37mmol), and continue to react at room temperature for 2 hours. The reaction solution was poured into saturated aqueous ammonium chloride solution (20mL), and extracted with ethyl acetate (20mL*3). The obtained organic phases were combined and washed with saturated brine (20mL), dried over anhydrous sodium sulfate, filtered, and the organic phase After the solvent was distilled off under reduced pressure, the resulting residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 2:1-1:1) to obtain light yellow liquid 30B (4.58 g, yield 83%).
References:
WO2022/214053,2022,A1 Location in patent:Page/Page column 50
616-47-7
763 suppliers
$10.00/100g
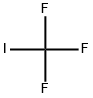
2314-97-8
186 suppliers
$40.00/25g
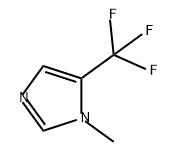
81769-70-2
0 suppliers
inquiry

81769-69-9
19 suppliers
inquiry
70631-94-6
1 suppliers
inquiry