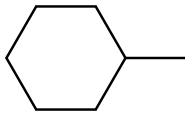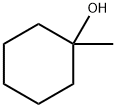
1-Methylcyclohexanol synthesis
- Product Name:1-Methylcyclohexanol
- CAS Number:590-67-0
- Molecular formula:C7H14O
- Molecular Weight:114.19
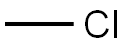
74-87-3
222 suppliers
$18.50/48622
108-94-1
584 suppliers
$12.00/50g

590-67-0
221 suppliers
$10.00/1g
Yield:590-67-0 115 g
Reaction Conditions:
Stage #1: methylene chloride in tetrahydrofuran at 0; for 2 h;
Stage #2: with magnesium;ethylene dibromide in tetrahydrofuran at 35; for 1 h;Inert atmosphere;
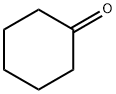
Stage #3: cyclohexanonewith lithium chloride in tetrahydrofuran at 0 - 10; for 1.5 h;Inert atmosphere;
Steps:
1.1 Example 1
The specific experimental procedures of this example are as follows. first step: To a 2 L four-necked reaction flask was added THF (800 g), and the internal temperature was lowered to 0 ° C by an ethanol dry ice bath, and methyl chloride (200 g) was controlled below 0 ° C, and the mixture was added dropwise after 2 hours, and the solution was used.Magnesium turnings (55.9g) and THF (80g) were added to a 5L four-neck reaction flask, protected by nitrogen, the internal temperature was raised to 60 ° C, and the drops were added with 1,2-dibromoethane. A large number of bubbles appeared in the reaction flask. The regenerative heat is reversing, the reaction liquid is grayed out, and finally it is black.The temperature was lowered to 35 ° C, the temperature of the system was stabilized at 35 ° C ± 5 ° C, and a solution of methyl chloride in THF was added dropwise, and the mixture was dropped in about 1 hour.Then the system was cooled to 0 ° C, 15 g of anhydrous lithium chloride was added, and cyclohexanone (150 g) was added dropwise, and the dropping temperature was controlled at 0 ± 5 ° C. The reaction liquid was black at the beginning, and then slowly grayed out. Become sticky.After 30 minutes of dropwise addition, the temperature was raised to 10 ± 5 ° C, and the reaction was maintained at 10 ± 5 ° C for 1 hour (central control 1, GC detection, raw material content A 25% aqueous solution of ammonium chloride was placed, and ammonium chloride (81.8 g) was added to water (245.5 g) and stirred until a clear liquid.An aqueous solution of ammonium chloride was added dropwise, and the temperature was controlled to be 20 ° C or lower.A white solid appeared in the reaction flask and was suction filtered.The filter cake was beaten twice with 225 g of ethyl acetate each time and rinsed with 25 g of ethyl acetate. The organic phases were combined and dried over anhydrous sodium sulfate.Concentration under reduced pressure at 30 ° C gave 138 g (yield: 2, dissolved GC The water was distilled, the oil bath was 90 ° C, and the top temperature was 74 to 78 ° C to start the pre-fraction.After the top temperature was stabilized at 78 ° C for 5 minutes, the main fraction was taken to obtain 115 g of 1-methylcyclohexanol, a colorless transparent liquid (central control 3, main content GC purity > 98%).
References:
CN109485565,2019,A Location in patent:Paragraph 0030-0038
![Benzene, 1-methoxy-4-[[(1-methylcyclohexyl)oxy]methyl]-](/CAS/20210305/GIF/1020186-18-8.gif)
1020186-18-8
0 suppliers
inquiry

590-67-0
221 suppliers
$10.00/1g
![1-methyl-7-oxabicyclo[4.1.0]heptane](/CAS/GIF/1713-33-3.gif)
1713-33-3
57 suppliers
$28.00/250mg

590-67-0
221 suppliers
$10.00/1g
16737-30-7
1 suppliers
inquiry

590-67-0
221 suppliers
$10.00/1g