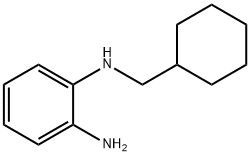
1-N-(cyclohexylmethyl)benzene-1,2-diamine synthesis
- Product Name:1-N-(cyclohexylmethyl)benzene-1,2-diamine
- CAS Number:163618-44-8
- Molecular formula:C13H20N2
- Molecular Weight:204.31
Yield:163618-44-8 19%
Reaction Conditions:
with sodium iodide in N,N-dimethyl-formamide at 23; for 24 h;Inert atmosphere;
Steps:
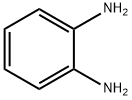
6.3.1. N-Cyclohexylmethyl-1,2-phenylenediamine (12)
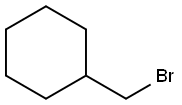
A solution of 1,2-phenylenediamine 11 (5.0 g; 0.046 mol), cyclohexylmethyl bromide (8.1 g; 0.046 mol) and sodium iodide (7.0 g; 0.046 mol) in dry dimethylformamide (250 mL) was stirred at 23 °C for 24 h under a nitrogen atmosphere. The solution was diluted with water (200 mL) and extracted with ethyl acetate (4 × 200 mL); the combined organic extracts were washed with brine (500 mL), dried and concentrated in vacuo to an oil, which was purified by flash chromatography (eluting with cyclohexane-AcOEt 8:2) to give the title compound as a white solid (1.8 g). Y = 19% Mp 72-75 °C. TLC cyclohexane-AcOEt 8:2, Rf = 0.55. IR (nujol): 3400, 3371 and 3271 (NH2 and NH) cm-1. 1H NMR: 6.83 (m, 1H); 6.75-6.62 (m, 3H); 3.32 (br s, 3H); 2.95 (d, 2H); 1.86 (bd, 2H); 1.8-1.55 (m, 4H); 1.35-0.95 (m, 5H). MS: m/z = 204 [M]+, 205 [M+H]+
References:
Roberts, Karen;Ursini, Antonella;Barnaby, Robert;Cassar, Paolo G.;Corsi, Mauro;Curotto, Giovanni;Donati, Daniele;Feriani, Aldo;Finizia, Gabriella;Marchioro, Carla;Niccolai, Daniela;Oliosi, Beatrice;Polinelli, Stefano;Ratti, Emiliangelo;Reggiani, Angelo;Tedesco, Giovanna;Tranquillini, Maria E.;Trist, David G.;Van Amsterdam, Franciscus T.M. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 14,p. 4257 - 4273] Location in patent:experimental part
13534-98-0
284 suppliers
$6.00/250mg
3218-02-8
155 suppliers
$21.00/1mL

163618-44-8
5 suppliers
inquiry