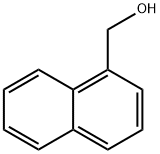
1-Naphthalenemethanol synthesis
- Product Name:1-Naphthalenemethanol
- CAS Number:4780-79-4
- Molecular formula:C11H10O
- Molecular Weight:158.2
Yield:-
Reaction Conditions:
with cyclohexene in propan-1-ol;water at 140; for 1 h;Micellar solution;Green chemistry;Reagent/catalyst;Time;
Steps:
General procedure for the transfer hydrogenation process
General procedure: Typically, to a stirred mixture (stirring rate 400 rpm) of the unsaturated hydrogen acceptor (0.893 mmol) and the hydrogen donor (3.572 mmol), calculated to make the combination of the acceptor and the donor 1.65 wt.% of the emulsion, was added in a preheated pressure tube (or a mini-autoclave) with a mixture of a surfactant (3.27 wt.%), a co-surfactant (usually 1-PrOH, 6.54 wt.%) and TDW (88.54 wt.%). In most cases, this operation formed transparent microemulsions that dispersed laser beams. To this mixture was added the immobilized palladium pre-catalyst (containing e.g., 0.067 mmol Pd). Following this addition the reaction vessel was heated up to the desired reaction temperature. After the desired reaction period the reaction mixture was cooled to 20 ◦C and the sol-gel material was filtered off. The filtrate was separated into two phases by addition of NaCl (2 g). The solid material was sonicated with hexane (8 ml) and the aqueous layer of the filtrate was extracted with hexane (2× 8 ml). The combined organic layers were dried (MgSO4) concentrated and analyzed by GC and 1H NMR. Some of the products were separated by column chromatography. The used catalyst was dried over P2O5 and reused in further runs
References:
Batarseh, Charlie;Nairoukh, Zackaria;Volovych, Iryna;Schwarze, Michael;Schomäcker, Reinhard;Fanun, Monzer;Blum, Jochanan [Journal of Molecular Catalysis A: Chemical,2013,vol. 366,p. 210 - 214]
66-77-3
388 suppliers
$7.00/10g

4780-79-4
244 suppliers
$6.00/5g
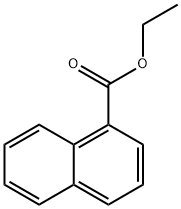
3007-97-4
69 suppliers
$23.00/1 g

4780-79-4
244 suppliers
$6.00/5g
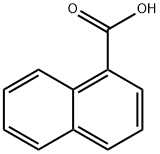
86-55-5
378 suppliers
$5.00/5g

4780-79-4
244 suppliers
$6.00/5g
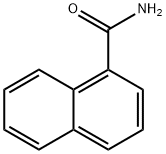
2243-81-4
108 suppliers
$10.00/1g

4780-79-4
244 suppliers
$6.00/5g