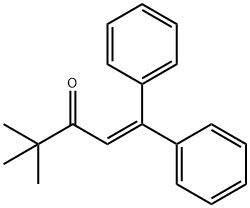
1-Penten-3-one, 4,4-dimethyl-1,1-diphenyl- synthesis
- Product Name:1-Penten-3-one, 4,4-dimethyl-1,1-diphenyl-
- CAS Number:844-39-3
- Molecular formula:C19H20O
- Molecular Weight:264.36
Yield:844-39-3 91%
Reaction Conditions:
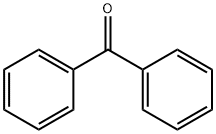
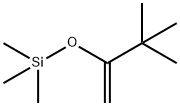
Stage #1: benzophenone;3,3-dimethyl-2-(trimethylsilyl)oxy-1-butenewith C40H61Al3O3 in acetonitrile at 0 - 5; for 72 h;Mukaiyama Aldol Addition;
Stage #2: with hydrogenchloride in water;acetonitrile at 20; for 3 h;Mukaiyama Aldol Addition;
Steps:
General procedure: Representative Procedure for Mukaiyama Aldol Reactions of Me2C C(OMe)OSiMe3 with Aryl Aldehydes Catalyzed by 1-5. To an oven-dried 10-mL vial equipped with a magnetic stirring bar and a septum was added acetonitrile (5 mL), 1 (0.03 mmol), and aldehyde (1.0 mmol). The mixture was stirred for 30 min at room temperature, followed by addition of Me2C C(OMe)OSiMe3 (1.2 mmol). When the reaction was judged to be complete by TLC, the reaction was quenched by adding 2 N HCl (3 mL) solution followed by stirring the reaction mixture for an additional 3 h at room temperature. The reaction mixture was extracted with methylene chloride and dried over Na2SO4, the solution was filtered and dried on a rotavap apparatus, and then the crude product was purified by silica gel column chromatography (EtOAc:hexanes = 1:9).
References:
Kim, So Han;Yoon, Sungwoo;Kim, Youngjo;Verkade, John G. [Phosphorus, Sulfur and Silicon and the Related Elements,2014,vol. 189,p. 1193 - 1206]
1522-15-2
1 suppliers
inquiry

844-39-3
0 suppliers
inquiry

95546-33-1
0 suppliers
inquiry

844-39-3
0 suppliers
inquiry
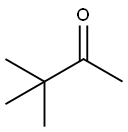
75-97-8
245 suppliers
$10.00/1g

844-39-3
0 suppliers
inquiry

5333-12-0
0 suppliers
inquiry

844-39-3
0 suppliers
inquiry