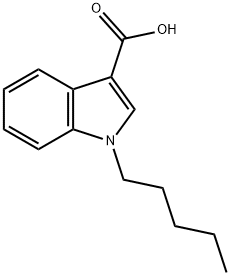
1-pentyl-1H-indole-3-carboxylic acid synthesis
- Product Name:1-pentyl-1H-indole-3-carboxylic acid
- CAS Number:727421-73-0
- Molecular formula:C14H17NO2
- Molecular Weight:231.29
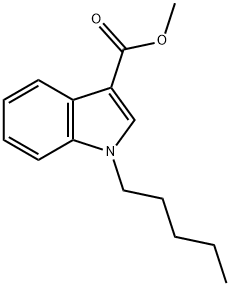
1338925-20-4

727421-73-0
Step 1. Compound 34 (2.34 g, 9.44 mmol) was dissolved in 45 mL of ethanol and 5 mL of aqueous solution of KOH (85%, 1.87 g, 28.33 mmol) was added slowly and dropwise. The reaction mixture was stirred at room temperature overnight. Subsequently, KOH (85%, 1 g) was added additionally and the mixture was heated to 75 °C and maintained for 4 hours. Upon completion of the reaction, the reaction solution was acidified with 1 M HCl (2 x 25 mL) and extracted with ether (3 x 25 mL). The organic phases were combined and residual water and ethanol were removed under reduced pressure using acetonitrile as an azeotrope (about 4 times). Further concentration under reduced pressure afforded 1-pentyl-1H-indole-3-carboxylic acid (2.11 g, 92% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 8.27-8.20 (m, 1H), 7.93 (s, 1H), 7.42-7.37 (m, 1H), 7.33-7.28 (m, 2H), 4.17 (t, J = 7.2 Hz, 2H), 1.94-1.85 (m, 2H), 1.38-1.30 ( m, 4H), 0.90 (t, J = 6.9Hz, 3H).

1338925-20-4
16 suppliers
$103.00/1mg

727421-73-0
46 suppliers
$39.00/1mg
Yield:727421-73-0 92%
Reaction Conditions:
Stage #1: methyl 1-pentyl-1H-indole-3-carboxylatewith potassium hydroxide in ethanol;water at 20 - 75;
Stage #2: with hydrogenchloride in ethanol;water;
Steps:
5.1.14. N-(Naphthalen-2-yl)-1-pentyl-1H-indole-3-carboxamide (18)
Step 1. To a solution of 34 (2.34 g, 9.44 mmol) in 45 mL EtOH was added dropwise a solution of KOH (85%, 1.87 g, 28.33 mmol) in 5 mL H2O, and the resulting mixture was stirred overnight at ambient temperature. Hereafter, an additional portion of KOH (85%, 1 g) was added and the mixture was successively heated to 75 °C for 4 h, acidified with 1 M HCl (2 × 25 mL) and extracted with Et2O ( × 3). Residual water and EtOH was removed in vacuo with CH3CN as azeotrope ( × 4), additional concentration in vacuo gave 1-pentyl-1H-indole-3-carboxylic acid (2.11 g, 92% yield). 1H NMR (400 MHz, CDCl3) δ 8.27-8.20 (m, 1H), 7.93 (s, 1H), 7.42-7.37 (m, 1H), 7.33-7.28 (m, 2H), 4.17 (t, J = 7.2 Hz, 2H), 1.94-1.85 (m, 2H), 1.38-1.30 (m, 4H), 0.90 (t, J = 6.9 Hz, 3H).
References:
Blaazer, Antoni R.;Lange, Jos H.M.;Van Der Neut, Martina A.W.;Mulder, Arie;Den Boon, Femke S.;Werkman, Taco R.;Kruse, Chris G.;Wadman, Wytse J. [European Journal of Medicinal Chemistry,2011,vol. 46,# 10,p. 5086 - 5098] Location in patent:experimental part
771-50-6
603 suppliers
$5.00/100mg
110-53-2
412 suppliers
$13.69/5g

727421-73-0
46 suppliers
$39.00/1mg
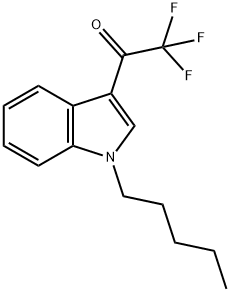
1430634-90-4
0 suppliers
inquiry

727421-73-0
46 suppliers
$39.00/1mg
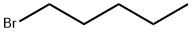
110-53-2
412 suppliers
$13.69/5g

727421-73-0
46 suppliers
$39.00/1mg
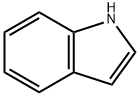
120-72-9
659 suppliers
$6.00/25g

727421-73-0
46 suppliers
$39.00/1mg