1-phenyltriazolo[4,5-c]pyridine synthesis
- Product Name:1-phenyltriazolo[4,5-c]pyridine
- CAS Number:129303-82-8
- Molecular formula:C11H8N4
- Molecular Weight:196.21
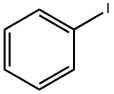
35826-31-4
21 suppliers
inquiry
![1-phenyltriazolo[4,5-c]pyridine](/CAS/20180723/GIF/129303-82-8.gif)
129303-82-8
7 suppliers
inquiry
Yield:129303-82-8 94%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water at 0 - 20; for 14 h;
Steps:
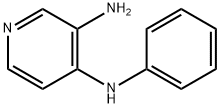
46.3 46.3 1 -Phenyl-1 H-El ,2,3]triazolo[4,5-c]pyridine
Compound 46.2 (500.0 mg; 2.683 mmol) was dissolved in hydrochloric acid (40.3 mL; 4.025 mmol) and cooled to 0 °C. Sodium nitrite (280.5 mg; 4.025 mmol), dissolved in water (5.0 mL), was added slowly while a colorless precipitate was formed. The suspension was stirred at 0 °C for 30 mm and then allowed to warm up to room temperature for 14 h. The reaction mixturewas diluted with saturated aqueous NaHCO3-solution and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo. The residue was purified by flash chromatography (CombiFlashRF 200). Yield: 496 mg (94%) beige solid;LC/MS, Rt: 1.71 mm; (M+H) 197.1.
References:
WO2017/20981,2017,A1 Location in patent:Page/Page column 65; 66
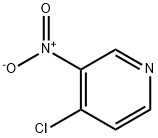
13091-23-1
343 suppliers
$14.14/1gm:
![1-phenyltriazolo[4,5-c]pyridine](/CAS/20180723/GIF/129303-82-8.gif)
129303-82-8
7 suppliers
inquiry

62-53-3
689 suppliers
$10.00/1g
![1-phenyltriazolo[4,5-c]pyridine](/CAS/20180723/GIF/129303-82-8.gif)
129303-82-8
7 suppliers
inquiry
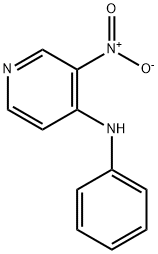
35750-90-4
5 suppliers
inquiry
![1-phenyltriazolo[4,5-c]pyridine](/CAS/20180723/GIF/129303-82-8.gif)
129303-82-8
7 suppliers
inquiry
![1H-1,2,3-TRIAZOLO[4,5-C]PYRIDINE](/CAS/GIF/273-05-2.gif)