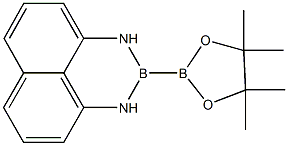
1-pinacolato-2-(1,8)diamo-naphthalenylborane synthesis
- Product Name:1-pinacolato-2-(1,8)diamo-naphthalenylborane
- CAS Number:1214264-88-6
- Molecular formula:C16H20B2N2O2
- Molecular Weight:293.96
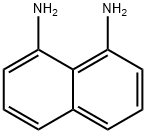
479-27-6

73183-34-3

1214264-88-6
General Steps: EXAMPLE: A 50 mL reaction flask was placed on a magnetic stirrer and pinacol ester of bisboronic acid (1.0 mmol, 1.0 eq.), 1,8-diaminonaphthalene (1.0 mmol, 1.0 eq.), and sodium methanol (1.0 mmol, 1.0 eq.) were added sequentially. After purging the reaction system with nitrogen three times under nitrogen protection, 10 mL of methanol was added as solvent. The reaction mixture was heated to 70 °C and stirred continuously for 4 hours. After completion of the reaction, the solvent methanol was removed by rotary evaporator. The crude product was purified by column chromatography to afford the target product 1-pinacol-2-(1,8)naphthalenediamine biboronate in 90% yield.

479-27-6
377 suppliers
$6.00/10g

73183-34-3
587 suppliers
$6.00/5g

1214264-88-6
57 suppliers
inquiry
Yield: 90%
Reaction Conditions:
with sodium methylate in methanol at 70; for 4 h;Inert atmosphere;
Steps:
Example: 50mL reaction flask was placed in a magnetic stirrer, followed by adding borate dicarboxylate ester (1.0 mmol, 1.0 eq.), 1,8-diaminonaphthalene (1.0 mmol, 1.0 eq.) And sodium methoxide (1.0 mmol, 1.0 eq.), After nitrogen was purged three times, 10 mL of solvent methanol was added under a nitrogen stream, and the reaction was heated to 70 ° C. After stirring for four hours, The reaction ends. After the solvent was removed by distilling off the toluene, The asymmetric bisphosphonization reagent Bpin-Bdan was isolated by column chromatography, and the yield was 90%
References:
Xijing College;Ding Siyi;Zhao Yuzhen;Ma Qiang;Tian Shaopeng;Liu Jiaxin;Yu Fei CN106946916, 2017, A Location in patent:Paragraph 0012
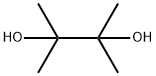
76-09-5
397 suppliers
$8.19/250mg
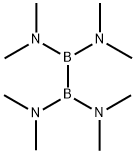
1630-79-1
99 suppliers
$23.00/5g

479-27-6
377 suppliers
$6.00/10g

1214264-88-6
57 suppliers
inquiry

76-09-5
397 suppliers
$8.19/250mg

479-27-6
377 suppliers
$6.00/10g

1214264-88-6
57 suppliers
inquiry