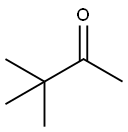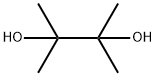
Pinacol synthesis
- Product Name:Pinacol
- CAS Number:76-09-5
- Molecular formula:C6H14O2
- Molecular Weight:118.17
62-53-3
685 suppliers
$10.00/1g
502630-89-9
12 suppliers
inquiry

76-09-5
395 suppliers
$10.00/1g
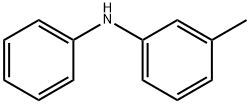
1205-64-7
256 suppliers
$5.00/5g
Yield:1205-64-7 5 - 9 %Chromat. ,76-09-5 20 - 25.5 %Chromat.
Reaction Conditions:
with potassium phosphate;tris-(dibenzylideneacetone)dipalladium(0);2′-(diphenylphosphino)-N,N′-dimethyl-(1,1′-biphenyl)-2-amine in 1,2-dimethoxyethane at 100; for 21 h;Buchwald-Hartwig coupling;
Steps:
1 EXAMPLE 1
This example illustrates the tandem Ir-catalyzed borylation and catalytic amination process. [0064] 3-Aminoboronic acids and esters as shown below are of interest as evidenced by the large number of derivatives synthesized, and by several patents, which note their activity as O-lactamase inhibitors (See, for example, Shoichet et al., WO0035905). Few in number, however, are 1, 3, 5-aminoboronic acids and esters (about 25 compounds by SCIFINDER SCHOLAR). Such substrates may prove useful for further derivatization as they can possess three unique sites for diversity. Furthermore, these compounds may prove ideal as scaffolds for combinatorial libraries. The boronic acid or ester can be transformed into a myriad of functionalities including aryl or vinyl via the Suzuki-Miyuara coupling (Miyaura and Suzuki, Chem. Rev. 95: 2457-2483 (1995); Suzuki, J. Organomet. Chem. 576: 147-168 (1999); Miyaura, In Advances in Metal-Organic Chemistry: Liebeskind, Ed.: JAI: London,; Vol. 6, pp. 187-243 (1998)). If R is a halogen, then there exists a multitude of coupling opportunities (See, for examples, Metal-catalyzed Cross-coupling Reactions; Diederich and Stang, eds.: Wiley: Wienheim, 1998). [0066] Recently, a catalytic aromatic C-H activation/borylation reaction utilizing Ir- or Rh-catalysts was developed. The process is high yielding, functional group tolerant (alkyl, halo, carboxy, alkoxy, and protected amino), chemoselective (1,3-substited arenes give only the 5-boryl product), and efficient (Iverson and Smith, J. Am. Chem. Soc. 121: 7696-7697 (1999); Cho et al., J. Am. Chem. Soc. 122: 12868-12869 (2000); Tse et al., Org. Lett. 3: 2831 (2001); Chao et al., Science 295: 305-308 (2002)). Furthermore, the process allows for the direct construction of aryl boronic esters from hydrocarbon feedstocks without going through an aryl halide. Scheme 2 depicts a prototypical borylation reaction: borylation of benzene using (Ind)Ir(COD)(2 mol %), dppe (2 mol %). The borane of choice is pinacolborane (HBPin). A variety of Ir(I) catalysts can be used, including [Ir(COD)Cl]2, Ir(Indenyl)(C2H4)2, Ir(Indenyl)dppe, and (Indenyl)Ir(COD), in the presence of 2 mol equivalents of PMe3 or 1 mol equivalent of a bidentate ligand like dmpe or dppe. The catalyst system of choice is (Indenyl)Ir(COD), dppe or dmpe (2 mol % each) because of it's cleanness of reaction and efficient TOF (24 h-1 with benzene). The reaction can be run in the neat arene or in inert solvents (e.g. cyclohexane). During our studies into tandem borylation/Suzuki coupling, we noted difficulties with the hydrolysis of the boronic ester functionality (Bpin). The robustness of the BPin group suggested that, perhaps, the pinacol might serve as a protecting group for the boron. Thus, it was deemed of interest to explore other catalytic transformations in the presence of the BPin group. One such transformation is the Buchwald-Hartwig amination of aryl halides (See, for example; Wolfe et al.,. J. Org. Chem. 65: 1158 (2000); Hartwig et al., J. Org. Chem. 64: 5575 (1999); Wolfe and Buchwald, Angew. Chem. Int. Ed. 38: 2413 (1999)). Initially, the reaction was attempted on pure 1-chloro-3-methylphenyl-5-BPin. As shown in Scheme 3 (Buchwald-Hartwig coupling of 1-chloro-3-methylphenyl-5-BPin with aniline), application of Buchwalds protocol proceeded cleanly to give the desired cross-coupling product in 64.7% and 63.8% yield. The use of PtBu3 improved the yield to 78.8%. Unfortunately, initial attempts to perform the reaction in the “one-pot” protocol were unsuccessful. Table 1 summarizes the results. In all cases where K3PO4.nH2O was used, a significant amount of pinacol was observed by GC-FID (Entries 1-5). While this is indicative of reaction of the BPin group and is most likely a by-product of Suzuki coupling (in this case, dimerization or oligiomerization of the starting material), no dimers or oligiomers were isolated. Noteworthy, is the formation of the desired product, albeit in low yield (10% GC-FID ratio), using K3PO4.nH2O and PtBu3 when all other attempts using the base failed. With anhydrous K3PO4, results were better (Entries 6-9). Most importantly, no pinacol was formed in these reactions. Changing the base or increasing catalyst loading did not improve the results. The use of PtBu3 led to the best results and after 4 days at 100° C., 34.4% of the desired product was isolated (Entry 10). This result, however, falls short of the reaction performed on pure material and shows that the by-products from the Ir-catalyzed borylation are not completely innocuous. As was previously mentioned, a potential source of concern is the presence of free bidentate phosphines after the borylation, which may interfere with subsequent reactions. In the tandem Suzuki reactions, an aryl chloride was successfully coupled only when dmpe was used as the Ir ligand. Thus, the tandem borylation/Buchwald-Hartwig amination reaction of the present invention was attempted using the (Ind)Ir(COD)/dmpe precatalyst. Gratifyingly, this protocol gave the desired aminoaryl boronic ester is an overall yield of 70.8% and 74.8% from 3-chlorotoluene. This one-pot borylation/Buchwald-Hartwig amination process of the present invention is shown in Scheme 4. Again, it was preferable to run the reaction under anhydrous conditions to substantially avoid Suzuki coupling. If one performs the reaction in a stepwise fashion, the overall yield is 60.1%. Thus, it appears that the tandem approach affords the product in significantly better yield. A series of substrates and amines were subjected to the tandem borylation/Buchwald-Hartwig (B-H) amination protocol of the present invention, as shown in Table Thus, both electron-rich and electron-poor haloaryl boronic esters can be aminated in moderate to good yields using this protocol. Thus, for example, borylation of 3-chlorotoluene followed by amination with morpholine using Pd2dba3 and PtBu3 gives the desired aminoaryl boronic ester in 73.4% yield. Borylation of 3-trifluoromethyl toluene followed by amination with aniline using Pd2dba3 with either the Buchwald biaryl phosphine or Hartwig PtBu3 ligand also gives the corresponding aminoaryl boronic ester in good yield (Entry 1; 68.7% for the former and 69.7% for the latter). Contrariwise, amination of the same aryl boronic ester with morpholine was not as successful (Entry 6; 47.6%). For this reaction, a compound (GC-FID ratio to product is 7.7 to 92.3) tentatively identified by GC-MS and 1H NMR as the aminated dimer was isolated. The structure of the aminated dimer is The aminated dimmer is believed to be the result of amine induced Suzuki coupling of the aryl boronic ester with another molecule of itself followed by Pd-catalyzed amination of the remaining chloride group. A similar dimerization was observed for Entry 5 and is presumably formed in a similar manner. As the initialdimer is bifunctional (i.e., it has a chloro and a BPin group), further oligiomerization is likely and thus may account for the poor yield in these cases. The Suzuki coupling is facilitated by electron-poor aryl boronic esters (Entries 1, 3, 5, 6), which will activate the boron towards nucleophilic attack by a base and by a stronger base [protonated pKa (morpholine)=8.36; pKa (aniline)=0.78] (Entries 4-6). It is, therefore, likely that the low yields in Table 2 are attributable to competing Suzuki oligiomerization. The successful tandem reaction using (Ind)Ir(COD), dppe for borylation for Entry 1 was somewhat unexpected. Recall that for 3-chlorotoluene, the use of dppe inhibited the subsequent amination. It may simply be that the more activated aryl halides are readily aminated and do not require highly active Pd-catalysts. [0072] While this coupling is remarkable, the usefulness of the products for further transformations could be called in question. In particular, could the boronic ester functionality be coupled to give an aminobiaryl (Scheme 1, lower reaction) or would the amine undergo Buchwald-Hartwig amination to give a trisubstituted amine (Scheme 1, upper reaction). To address this issue, pure 1-N-phenyl-3-methylphenylboronic ester was subjected to coupling with 3-chlorobromobenzene. Gratifyingly, the desired biaryl was obtained in 69% yield. In addition to the selectivity of the coupling, the product would not be easily accessible following an alternative route; namely, Suzuki coupling followed by amination because there would be an issue as to which chloro group would be functionalized.
References:
US2004/24237,2004,A1 Location in patent:Page 7-11
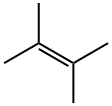
563-79-1
143 suppliers
$29.00/5mL

76-09-5
395 suppliers
$10.00/1g
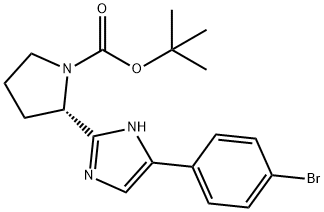
1007882-04-3
59 suppliers
inquiry

73183-34-3
584 suppliers
$6.00/5g

76-09-5
395 suppliers
$10.00/1g
![(S)-2-[5-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1H-imidazol-2-y](/CAS/GIF/1007882-12-3.gif)
1007882-12-3
37 suppliers
inquiry
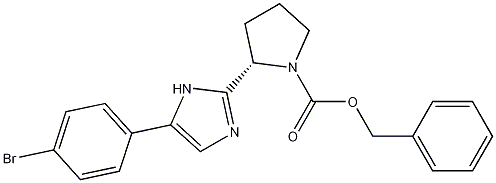
1007882-39-4
0 suppliers
inquiry

73183-34-3
584 suppliers
$6.00/5g

76-09-5
395 suppliers
$10.00/1g
![1-Pyrrolidinecarboxylic acid, 2-[5-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1H-imidazol-2-yl]-, phenylmethyl ester, (2S)-](/CAS/20210305/GIF/1007882-17-8.gif)
1007882-17-8
0 suppliers
inquiry