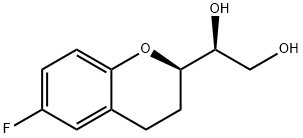
(1’S,2R)-2-(1’,2’-Dihydroxyethyl)-6-fluorochromane synthesis
- Product Name:(1’S,2R)-2-(1’,2’-Dihydroxyethyl)-6-fluorochromane
- CAS Number:303176-43-4
- Molecular formula:C11H13FO3
- Molecular Weight:212.22
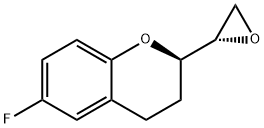
129050-26-6
36 suppliers
$115.00/5mg

303176-43-4
14 suppliers
$140.00/50mg
Yield:> 99 % ee
Reaction Conditions:
Stage #1: (2R)-6-fluoro-2-[(2S)-oxiran-2-yl]-3,4-dihydro-2H-chromenewith oxygen;acetic acid;[(S,S)-N,N’-bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]cobalt(II) in tert-butyl methyl ether;toluene at 20; for 1 h;
Stage #2: with water in tert-butyl methyl ether;toluene at 25; for 24 h;Product distribution / selectivity;
Steps:
14.A
the catalyst (S,S)-(-)-N(N'-Bis-(3,5-di-fer/-butylsalicylidene)-l,2-cyclo-hexane- diamino-cobalt-(H) (12.0 mg) was dissolved in toluene (0.1 ml) and treated with AcOH (6.6 mg). The solution was allowed to stir at rt open to air for 1 h and was concentrated in vacuo to obtain a crude brown solid.The resulting catalyst residue was dissolved in (2R)-6-fluoro-2-[(2S)-oxiran-2-yl]-3,4- dihydro-2H-chromene (0.97 g), (2R)-6-fluoro-2-[(2R)-oxiran-2-yl]-3,4-dihydro-2H- chromene (0.97 g) [(R,R)-,(R,S)-epoxide diastereoisomeric mixture)] and MTBE (2.36 ml)
References:
WO2008/64826,2008,A1 Location in patent:Page/Page column 27-28
![1,2-Ethanediol, 1-[(2R)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl]-, 2-acetate, (1S)-](/CAS/20210305/GIF/1345202-52-9.gif)
1345202-52-9
0 suppliers
inquiry

303176-43-4
14 suppliers
$140.00/50mg
![2H-1-Benzopyran, 2-[(4R)-2,2-dimethyl-1,3-dioxolan-4-yl]-6-fluoro-3,4-dihydro-](/CAS/20210305/GIF/1030385-06-8.gif)
1030385-06-8
0 suppliers
inquiry
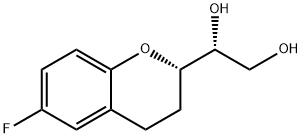
303176-39-8
13 suppliers
$255.00/100mg
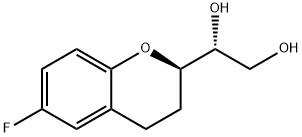
303176-45-6
14 suppliers
$140.00/100mg

303176-43-4
14 suppliers
$140.00/50mg

303176-38-7
0 suppliers
inquiry

303176-43-4
14 suppliers
$140.00/50mg
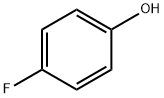
371-41-5
533 suppliers
$8.00/25g

303176-43-4
14 suppliers
$140.00/50mg