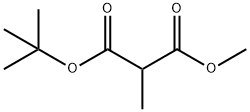
1-tert-Butyl 3-methyl 2-methylmalonate synthesis
- Product Name:1-tert-Butyl 3-methyl 2-methylmalonate
- CAS Number:132180-14-4
- Molecular formula:C9H16O4
- Molecular Weight:188.22
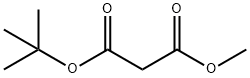
42726-73-8

74-88-4

132180-14-4
To a 100 mL round-bottom flask equipped with a magnetic stirrer was added tert-butyl methyl malonate (1920 mg, 11.0 mmol) and anhydrous DMF (25 mL). Sodium hydride (60% oil suspension, 446 mg, 11.0 mmol) was slowly added to this solution at 0 °C. The reaction mixture was stirred continuously for 30 hours at 0 °C. Subsequently, iodomethane (670 μL, 11.0 mmol) was added dropwise to the reaction system at 0 °C and the mixture was transferred to room temperature for continued stirring for 18 hours. Upon completion of the reaction, the reaction was quenched by adding deionized water to the mixture and extracted with dichloromethane (3 × 25 mL). The organic phases were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 90:10) to afford the target compound, 3-methyl-2-methylmalonyl-1-tert-butyl ester (1597 mg, 77%), as a colorless oil.1H NMR (400 MHz, CDCl3) δ 3.73 (s, 3H), 3.35 (q, J = 7.3 Hz, 1H), 1.46 (s, 9H), 1.38 (d, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.9, 169.2, 81.6, 52.2, 47.0, 27.8, 13.5.
Yield:132180-14-4 77%
Reaction Conditions:
Stage #1: methyl t-butyl malonatewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 30 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 18 h;
Steps:
4 4.4 1-tert-Butyl 3-methyl 2-methylmalonate 3
A 100 mL round-bottom flask equipped with a stirring bar was charged with compound 7 (1920 mg, 11.0 mmol) and DMF (25 mL). To the solution, sodium hydride (60% oil suspension, 446 mg, 11.0 mmol) was added. The reaction was allowed to stir at 0 °C for 30 h. To the mixture, methyl iodide (670 μL, 11.0 mmol) was added at 0 °C and the mixture was stirred at room temperature for 18 h. To the reaction mixture, H2O was added, and the mixture was extracted with CH2Cl2 (3x), dried over MgSO4, and concentrated in vacuo. The remaining residue was purified by silica gel column chromatography (hexanes/AcOEt = 90:10) to afford the desired product 3 (1597 mg, 77%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 3.73 (s, 3H), 3.35 (q, J = 7.3 Hz, 1H), 1.46 (s, 9H), 1.38 (d, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.9, 169.2, 81.6, 52.2, 47.0, 27.8, 13.5.
References:
Kanemitsu, Takuya;Furukoshi, Saeka;Miyazaki, Michiko;Nagata, Kazuhiro;Itoh, Takashi [Tetrahedron Asymmetry,2015,vol. 26,# 4,p. 214 - 218]
40052-13-9
240 suppliers
$6.00/5g

132180-14-4
4 suppliers
inquiry