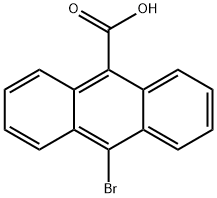
10-broMoanthracene-9-carboxylic acid synthesis
- Product Name:10-broMoanthracene-9-carboxylic acid
- CAS Number:6929-81-3
- Molecular formula:C15H9BrO2
- Molecular Weight:301.13
723-62-6
331 suppliers
$6.00/100mg

6929-81-3
29 suppliers
$70.00/250mg
Yield: 90%
Reaction Conditions:
with bromine;acetic acid at 60; for 4 h;
Steps:
1
9-anthracenecarboxylic acid (7.40 g, 3.33 mmol), acetic acid (AcOH, 0.6 L), bromine (Br 2, 1.8 ml) were placed in a reaction vessel and stirred at 60 ° C. for 4 hours for reaction .After completion of the reaction, the reaction product was added to 1.0 L of water saturated with potassium carbonate (K 2 CO 3) and stirred. The resulting solution was neutralized with concentrated hydrochloric acid with sufficient stirring to give a bright yellow precipitate. While thoroughly washing the precipitate with water, it was collected by filtration and dried under reduced pressure to obtain yellow powder 1 (10-bromoanthracene-9-carboxylic acid, 9.0 g) (yield: 90%).
References:
TOYOTA CENTRAL R&D LABS INCORPORATED;MOTEGI, HIROFUMI;SHICHI, AKIRA;MUKAE, YUSUKE;IDOTA, YOSHINORI JP2016/222632, 2016, A Location in patent:Paragraph 0035-0037

124-38-9
134 suppliers
$175.00/23402
523-27-3
392 suppliers
$6.00/5g

6929-81-3
29 suppliers
$70.00/250mg

523-27-3
392 suppliers
$6.00/5g

6929-81-3
29 suppliers
$70.00/250mg

723-62-6
331 suppliers
$6.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

523-27-3
392 suppliers
$6.00/5g

6929-81-3
29 suppliers
$70.00/250mg