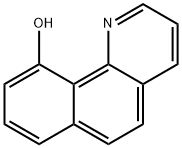
10-Hydroxybenzo[h]quinoline synthesis
- Product Name:10-Hydroxybenzo[h]quinoline
- CAS Number:33155-90-7
- Molecular formula:C13H9NO
- Molecular Weight:195.22
![10-bromobenzo[h]quinoline](/CAS/20200401/GIF/152583-10-3.gif)
152583-10-3
![10-Hydroxybenzo[h]quinoline](/CAS/GIF/33155-90-7.gif)
33155-90-7
10-Bromobenzo[h]quinoline (0.15 mmol, 39 mg) was added to an oven-dried screw-capped vial with carboxylic acid (0.3 mmol), silver carbonate (Ag-based, 0.165 mmol, 22 mg), potassium fluoride (0.15 mmol, 9 mg) and p-xylene (0.5 mL). The reaction mixture was stirred vigorously at 150 °C for 24 hours. After completion of the reaction, the mixture was cooled to room temperature and diluted with ethyl acetate. Subsequently, the solvent was removed by distillation under reduced pressure and the final product was purified by silica gel column chromatography.
![10-bromobenzo[h]quinoline](/CAS/20200401/GIF/152583-10-3.gif)
152583-10-3
2 suppliers
inquiry
![10-Hydroxybenzo[h]quinoline](/CAS/GIF/33155-90-7.gif)
33155-90-7
175 suppliers
$7.00/250mg
Yield:33155-90-7 95%
Reaction Conditions:
with ortho-methylbenzoic acid;potassium fluoride;silver carbonate in para-xylene at 150; for 24 h;Reagent/catalyst;Temperature;
Steps:
Experimental Procedures for the Hydroxylation
General procedure: 10-Bromobenzo[h]quinoline (0.15 mmol, 39 mg), carboxylic acid (0.3 mmol), silver carbonate (Ag based 0.165 mmol, 22mg), potassium fluoride (0.15 mmol, 9 mg), and p-xylene (0.5 mL) were added to an oven-dried screw-capped vial. The mixture was vigorously stirred at 150 oC for 24 h and then the resulting mixture was allowed to cool to room temperature and then diluted with ethyl acetate. Solvent was removed in vacuo, and the desired product was isolated by a silica gel column chromatography.
References:
Yoo, Kwangho;Park, Hyojin;Kim, Suyeon;Kim, Min [Tetrahedron Letters,2016,vol. 57,# 7,p. 781 - 783] Location in patent:supporting information
230-27-3
164 suppliers
$7.00/250mg
![10-Hydroxybenzo[h]quinoline](/CAS/GIF/33155-90-7.gif)
33155-90-7
175 suppliers
$7.00/250mg
13019-32-4
57 suppliers
$23.00/250mg
![10-Hydroxybenzo[h]quinoline](/CAS/GIF/33155-90-7.gif)
33155-90-7
175 suppliers
$7.00/250mg

408328-97-2
3 suppliers
inquiry
![10-Hydroxybenzo[h]quinoline](/CAS/GIF/33155-90-7.gif)
33155-90-7
175 suppliers
$7.00/250mg

408328-97-2
3 suppliers
inquiry
![Benzo[h]quinolin-10-amine](/CAS/20200611/GIF/186268-24-6.gif)
186268-24-6
0 suppliers
inquiry
![10-Hydroxybenzo[h]quinoline](/CAS/GIF/33155-90-7.gif)
33155-90-7
175 suppliers
$7.00/250mg