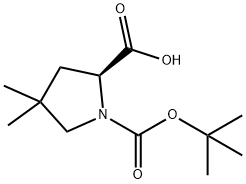
(S)-1-(tert-Butoxycarbonyl)-4,4-dimethylpyrrolidine-2-carboxylic acid synthesis
- Product Name:(S)-1-(tert-Butoxycarbonyl)-4,4-dimethylpyrrolidine-2-carboxylic acid
- CAS Number:1001353-87-2
- Molecular formula:C12H21NO4
- Molecular Weight:243.3

138423-86-6
52 suppliers
inquiry

1001353-87-2
66 suppliers
$113.00/100mg
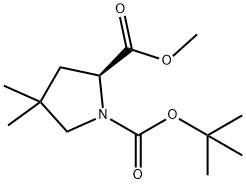
Yield:1001353-87-2 95%
Reaction Conditions:
with lithium hydroxide monohydrate in water at 0 - 20; for 2 h;Inert atmosphere;Sealed tube;
Steps:
15.2 the preparation of compound 15-3
To a solution of compound 15-2 (4.85 g, 18.86 mmol) in THF (40 mL) was added lithium hydroxide monohydrate aqueous solution (1.5 g, 20 mL) dropwise at 0 °C. At the end of the addition, the mixture was stirred at rt for 2.0 hrs. After the reaction was completed, the THF solvent was removed and 50 mL of water was added to the mixture. The resulting mixture was washed with EtOAc (30 mL x 3). The aqueous phase was adjusted to pH 1 with hydrochloric acid (1 M) and extracted with EtOAc (50 mL x 3). The combined organic layers were washed with brine, dried over anhydrous Na2S04 and concentrated in vacuo to give the title compound as a white solid (4.35 g, 95%). The compound was characterized by the following spectroscopic data: MS (ESI, pos.ion) mlz: 244.5 [M+H]+; 'H NMR (400 MHz, CD3OD) δ (ppm): 4.34-4.31 (m, 1H), 3.28-3.22 (m, 1H), 3.20-3.13 (m, 1H), 2.23-2.18 (m, 1H), 1.85-1.80 (m, 1H), 1.46 (s, 9H), 1.01-0.99 (m, 3H), 0.86-0.84 (m, 3H).
References:
WO2014/82380,2014,A1 Location in patent:Paragraph 00460; 00462
1001353-86-1
30 suppliers
$90.00/50mg

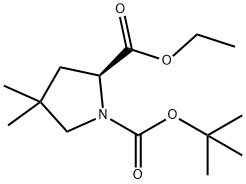
1001353-87-2
66 suppliers
$113.00/100mg

24424-99-5
871 suppliers
$13.50/25G

1001353-87-2
66 suppliers
$113.00/100mg
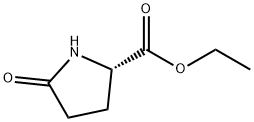
7149-65-7
236 suppliers
$15.00/25G

1001353-87-2
66 suppliers
$113.00/100mg
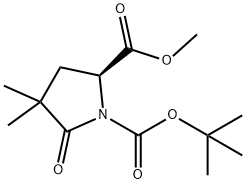
158392-74-6
21 suppliers
$155.00/25mg

1001353-87-2
66 suppliers
$113.00/100mg