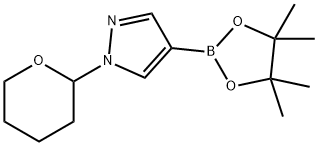
1-(2-Tetrahydropyranyl)-1H-pyrazole-4-boronic acid pinacol ester synthesis
- Product Name:1-(2-Tetrahydropyranyl)-1H-pyrazole-4-boronic acid pinacol ester
- CAS Number:1003846-21-6
- Molecular formula:C14H23BN2O3
- Molecular Weight:278.15
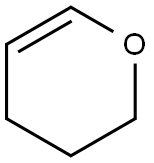
110-87-2
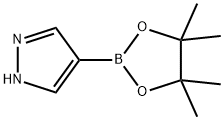
269410-08-4

1003846-21-6
The general procedure for the synthesis of 1-THP-4-pyrazoleboronic acid pinacol ester from 3,4-dihydro-2H-pyran and 4-pyrazoleboronic acid pinacol ester was as follows: 3,4-dihydro-2H-pyran (5.6 g, 67 mmol) and trifluoroacetic acid (1.17 g, 10.3 mmol) were added to a 4-(4,4,5,5-tetramethyl-1,3,2-dioxoborono pentan-2-yl)-1H-pyrazole (10.0 g, 51.5 mmol) in a solution of toluene (200 mL). The reaction mixture was heated to 90 °C and kept for 2 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was partitioned between ethyl acetate (200 mL) and saturated aqueous sodium bicarbonate solution (100 mL). The aqueous layer was further extracted with ethyl acetate (100 mL). The organic layers were combined, washed with saturated aqueous sodium chloride (100 mL), dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. Purification by silica gel column chromatography (elution gradient: 10% to 50% petroleum ether solution of ethyl acetate) afforded the target product 1-THP-4-pyrazoleboronic acid pinacol ester as a white solid. Yield: 13.4 g, 48.2 mmol, 94% yield.1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 7.83 (s, 1H), 5.41 (dd, J = 9.5, 2.5 Hz, 1H), 4.01-4.08 (m, 1H), 3.65-3.74 (m, 1H), 1.98- 2.18 (m, 3H), 1.60-1.76 (m, 3H), 1.32 (s, 12H).

110-87-2
543 suppliers
$10.00/1g

269410-08-4
397 suppliers
$5.00/1g

1003846-21-6
149 suppliers
$6.00/250mg
Yield:1003846-21-6 94%
Reaction Conditions:
with trifluoroacetic acid in toluene at 90; for 2 h;
Steps:
8.1 Step 1. Synthesis of 1-(tetrahydro-2H-pyran-2-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (C17)
3,4-Dihydro-2H-pyran (5.6 g, 67 mmol) and trifluoroacetic acid (1.17 g, 10.3 mmol) were added to a solution of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H- pyrazole (10.0 g, 51.5 mmol) in toluene (200 mL), and the reaction mixture was heated to 90 °C for 2 hours. After cooling to room temperature, the reaction mixture was partitioned between ethyl acetate (200 mL) and saturated aqueous sodium bicarbonatesolution (100 mL), and the aqueous layer was extracted with ethyl acetate (100 mL). The combined organic layers were washed with saturated aqueous sodium chloride solution (100 mL), dried over sodium sulfate, filtered, and concentrated in vacuo. Silica gel chromatography (Gradient: 10% to 50% ethyl acetate in petroleum ether) provided the product as a white solid. Yield: 13.4 g, 48.2 mmol, 94%. 1H NMR (400 MHz, CDCl3) 7.94 (s, 1H), 7.83 (s, 1H), 5.41 (dd, J=9.5, 2.5 Hz, 1H), 4.01-4.08 (m, 1H), 3.65-3.74(m, 1H), 1.98-2.18 (m, 3H), 1.6-1.76 (m, 3H), 1.32 (s, 12H).
References:
PFIZER INC.;GALATSIS, Paul;HAYWARD, Matthew Merrill;HENDERSON, Jaclyn;KORMOS, Bethany Lyn;KURUMBAIL, Ravi G;STEPAN, Antonia Friederike;VERHOEST, Patrick Robert;WAGER, Travis T.;ZHANG, Lei WO2014/1973, 2014, A1 Location in patent:Page/Page column 60; 61; 62
1040377-02-3
47 suppliers
$64.00/100mg

73183-34-3
586 suppliers
$6.00/5g

1003846-21-6
149 suppliers
$6.00/250mg

110-87-2
543 suppliers
$10.00/1g

1003846-21-6
149 suppliers
$6.00/250mg

73183-34-3
586 suppliers
$6.00/5g

1003846-21-6
149 suppliers
$6.00/250mg