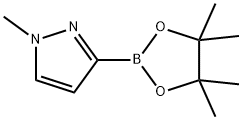
1-Methyl-3-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-Methyl-3-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:1020174-04-2
- Molecular formula:C10H17BN2O2
- Molecular Weight:208.07
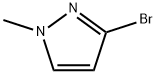
151049-87-5

73183-34-3

1020174-04-2
General procedure for the synthesis of 1-methylpyrazole-3-boronic acid pinacol ester from 3-bromo-1-methylpyrrole and bis(pinacolato)diboronic acid: firstly, compound 701 (3-bromo-1-methylpyrrole, 0.2 g, 1.24 mmol) was dissolved in 5 mL of 1,4-dioxane. Subsequently, bis(pinacolato)diboron (0.378 g, 1.49 mmol), potassium acetate (0.365 g, 3.72 mmol), and [1,1-bis(diphenylphosphino)ferrocene]palladium chloride dichloromethane complex (0.11 g, 0.124 mmol) were added to this solution. The reaction mixture was heated to 95 °C under nitrogen protection and kept at this temperature for 5 hours of reaction. After completion of the reaction, the mixture was cooled to room temperature. To the reaction mixture was added 15 mL of water, stirred for 1 hour and filtered to give 0.114 g of solid product, 1-methylpyrazole-3-boronic acid pinacol ester. The yield was 44.19%.

151049-87-5
166 suppliers
$21.00/1g

73183-34-3
587 suppliers
$6.00/5g

1020174-04-2
130 suppliers
$23.00/100mg
Yield:1020174-04-2 44.19%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 95; for 5 h;Inert atmosphere;
Steps:
46 Intermediate 702: 1-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
Intermediate 702: 1-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
Compound 701 (0.2 g, 1.24 mmol) was dissolved in 5 ml of 1,4-dioxane, added with 0.378 g (1.49 mmol) of bis(pinacolato)diboron, 0.365 g (3.72 mmol) of potassium acetate, and 0.11 g (0.124 mmol) of [1,1-bis(di-phenylphosphino)ferrocene]palladium chloride dichloromethane complex under the protection of nitrogen, heated to 95 °C and reacted for 5 h, and then cooled to room temperature. 15 mL of water was added, stirred for 1 h, and filtered to afford 0.114 g of solid. Yield: 44.19%.
References:
Beijing Forelandpharma Co. Ltd.;ZHANG, Xingmin;JI, Qi;WANG, Lei;GAO, Congmin;WANG, Ensi;DU, Zhenjian;GONG, Longlong;CHEN, Bo EP3072893, 2016, A1 Location in patent:Paragraph 0394; 0395
61676-62-8
323 suppliers
$10.00/5g
92525-10-5
146 suppliers
$16.00/100mg

1020174-04-2
130 suppliers
$23.00/100mg
269410-08-4
394 suppliers
$5.00/1g

74-88-4
357 suppliers
$15.00/10g

1020174-04-2
130 suppliers
$23.00/100mg