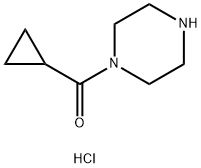
Piperazine, 1-(cyclopropylcarbonyl)-, Monohydrochloride synthesis
- Product Name:Piperazine, 1-(cyclopropylcarbonyl)-, Monohydrochloride
- CAS Number:1021298-67-8
- Molecular formula:C8H15ClN2O
- Molecular Weight:190.67
414910-15-9

1021298-67-8
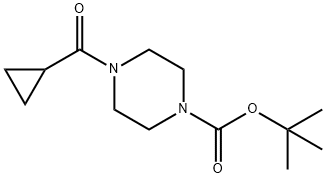
The general procedure for the synthesis of cyclopropyl (piperazin-1-yl)methanone hydrochloride from tert-butyl 4-(cyclopropanecarbonyl)piperazine-1-carboxylate was as follows: to a stirring solution of tert-butyl 4-(cyclopropanecarbonyl)piperazine-1-carboxylate (3.7 g, 14.5 mmol) in methanol (15 mL) was added slowly at 0 °C a hydrochloric acid/methanol solution (15 mL, 3M). After addition, the reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure to afford cyclopropyl (piperazin-1-yl)methanone hydrochloride (2.74 g, 100% yield) as an off-white solid. The product was characterized by 1H-NMR (400 MHz, DMSO-d6): δ 0.71-0.76 (m, 4H), 1.96-2.03 (m, 1H), 3.04-3.16 (m, 4H), 3.69-4.08 (m, 4H), 9.58 (s, 2H); LC-MS (ESI) m/z: 155 (M + 1)+.

414910-15-9
103 suppliers
$12.00/1mg

1021298-67-8
239 suppliers
$17.00/250mg
Yield: 100%
Reaction Conditions:
with hydrogenchloride in methanol at 0 - 20;
Steps:
17B
To a stirred mixture of compound tert-butyl 4-(cyclopropanecarbonyl)piperazine-1-carboxylate (3.7 g, 14.5 mmol) in methanol (15 mL) was added hydrochloride/methanol (15 mL, 3M)) at 0° C. After the addition, the mixture was allowed to stir at room temperature overnight. The mixture was concentrated to give cyclopropyl(piperazin-1-yl)methanone hydrochloride (2.74 g, yield 100%) as an off-white solid. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 0.71-0.76 (m, 4H), 1.96-2.03 (m, 1H), 3.04-3.16 (m, 4H), 3.69-4.08 (m, 4H), 9.58 (s, 2H); LC-MS (ESI) m/z: 155(M+1)+
References:
LEAD THERAPEUTICS, INC. US2010/35883, 2010, A1 Location in patent:Page/Page column 56

110-85-0
588 suppliers
$5.00/5 g
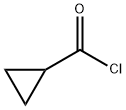
4023-34-1
371 suppliers
$12.00/5g

1021298-67-8
239 suppliers
$17.00/250mg