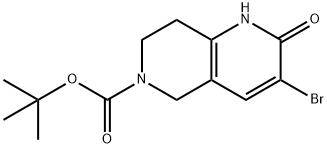
(Tert-butyl 3-bromo-2-oxo-1,5,7,8-tetrahydro-1,6-naphthyridine-6-carboxylate) synthesis
- Product Name:(Tert-butyl 3-bromo-2-oxo-1,5,7,8-tetrahydro-1,6-naphthyridine-6-carboxylate)
- CAS Number:1036381-92-6
- Molecular formula:C13H17BrN2O3
- Molecular Weight:329.19
1036381-91-5
36 suppliers
$198.00/100mg

24424-99-5
813 suppliers
$13.50/25G

1036381-92-6
5 suppliers
inquiry
Yield:1036381-92-6 63%
Reaction Conditions:
Stage #1: tert‐butyl 2‐oxo‐1,5,7,8‐tetrahydro‐1,6‐naphthyridine‐6(2H)‐carboxylatewith bromine;acetic acid at 20; for 3 h;
Stage #2: di-tert-butyl dicarbonatewith potassium carbonate in water at 20; for 14 h;
Steps:
G.1 Step 1:
To a solution of tert-butyl 2-oxo-1,5,7,8-tetrahydro-1,6-naphthyridine-6-carboxylate(275 mg,1.10 mmol)in acetic acid(2.2 mL)at 0 °C was added bromine(67.7 J.L,1.32 mmol)portionwise. The mixture was stirred at room temperature for 3 h,then concentrated underreduced pressure. The resulting residue was dissolved in chloroform(4 mL)and water(2.0 mL).To the solution was added Boc20(3H2 mg,1.43 mmol)and K2C03(304 mg,2.20 mmol). Themixture was stirred for 14 h at room temperature. The resulting precipitate was filtered,washed with diethyl ether,and dried under reduced pressure to give the first batch of the title compound.The mother liquor was poured into a separatory funnel,the organic later was separated,driedover Na2S04 and concentrated under reduced pressure. The resulting solid was washed withdiethyl ether,and then dried under reduced pressure to give the second batch of the titlecompound(228 mg total,63%)that required no further purification. 1H NMR(400 MHz,CDCl3,16 I 17 H)8 7.59(s,1H),4.32(s,2H),3.67(t,J = 5.8 Hz,2H),2.74(t,J = 5.8 Hz,2H),1.48(s,9H).
References:
WO2017/205536,2017,A2 Location in patent:Page/Page column 73; 74

1036381-91-5
36 suppliers
$198.00/100mg

1036381-92-6
5 suppliers
inquiry