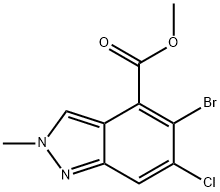
2H-Indazole-4-carboxylic acid, 5-bromo-6-chloro-2-methyl-, methyl ester synthesis
- Product Name:2H-Indazole-4-carboxylic acid, 5-bromo-6-chloro-2-methyl-, methyl ester
- CAS Number:1037841-35-2
- Molecular formula:C10H8BrClN2O2
- Molecular Weight:303.54
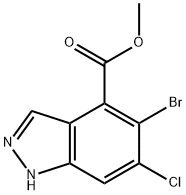
1037841-34-1
36 suppliers
$60.00/5mg
420-37-1
247 suppliers
$17.00/5g

1037841-35-2
0 suppliers
inquiry
Yield:1037841-35-2 82%
Reaction Conditions:
Stage #1: methyl 5-bromo-6-chloro-1H-indazole-4-carboxylate;trimethoxonium tetrafluoroborate in ethyl acetate at 10 - 35;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
57
Reference Example 57; methyl 5-bromo-6-chloro-2-methyl-2H-indazole-4-carboxylate [Show Image]; To a solution of methyl 5-bromo-6-chloro-1H-indazole-4-carboxylate (390 mg, 1.35 mmol) in ethyl acetate (27 mL) was added trimethyloxonium tetrafluoroborate (260 mg, 1.76 mmol) at room temperature, and the mixture was stirred for 2 hr. The reaction solution was diluted with ethyl acetate, washed with saturated aqueous sodium hydrogen carbonate solution, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (NH, ethyl acetate/hexane=10/90→40/60) to give the title compound (336 mg, yield 82%). 1H-NMR (CDCl3) δ: 4.00 (3H, s), 4.26 (3H, s), 7.58 (1H, s), 8.23 (1H, s), melting point: 141 - 142°C (recrystallized from ethyl acetate/hexane), MS (ESI+): 303 (M+H), elemental analysis: for C10H8N2BrClO2 Calculated (%): C, 39.57; H, 2.66; N, 9.23 Found (%): C, 39.48; H, 2.60; N, 9.25.
References:
EP2098513,2009,A1 Location in patent:Page/Page column 67-68