
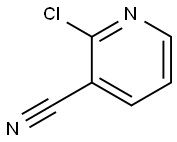
2-((tert-butoxycarbonyl)amino)thieno[2,3-b]pyridine-3-carboxylic acid synthesis
- Product Name:2-((tert-butoxycarbonyl)amino)thieno[2,3-b]pyridine-3-carboxylic acid
- CAS Number:1042441-74-6
- Molecular formula:C13H14N2O4S
- Molecular Weight:294.33

124-38-9
134 suppliers
$214.00/14L
![tert-butyl (3-bromothieno[2,3-b]pyridin-2-yl)carbamate](/CAS/20180703/GIF/1042441-73-5.gif)
1042441-73-5
3 suppliers
inquiry
![2-((tert-butoxycarbonyl)amino)thieno[2,3-b]pyridine-3-carboxylic acid](/CAS/20180703/GIF/1042441-74-6.gif)
1042441-74-6
4 suppliers
inquiry
Yield:1042441-74-6 82%
Reaction Conditions:
Stage #1: tert-butyl (3-bromothieno[2,3-b]pyridin-2-yl)carbamatewith n-butyllithium in tetrahydrofuran at -78; for 1 h;
Stage #2: carbon dioxide in tetrahydrofuran;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;
Steps:
5
Reference Example 5 2-[(tert-butoxycarbonyl)amino]thieno[2,3-b]pyridine-3-carboxylic acid [Show Image] A solution of tert-butyl (3-bromothieno[2,3-b]pyridin-2-yl)carbamate (1.12 g, 3.40 mmol) obtained in Reference Example 4 in THF (11 mL) was cooled to -78°C, 1.6 M n-butyllithium hexane solution (4.7 mL, 7.48 mmol) was added dropwise, and the mixture was stirred at the same temperature for 1 hr. Dry carbon dioxide was blown into the obtained suspension until the suspended solid was disappeared. 1N Hydrochloric acid (7.5 mL) was added to the reaction mixture and the resulting solid was collected by filtration. The obtained solid was suspended in water and collected by filtration, the obtained solid was suspended in acetonitrile and collected by filtration, and the obtained solid was suspended in diethyl ether and collected by filtration to give the title compound (815 mg, yield 82%). melting point 199-201°C EI(pos) 295 [M+]+ 1H NMR (DMSO-d6) δ1.54 (9H, s), 7.46 (1H, dd, J = 4.9 Hz, 8.1 Hz), 8.41-8.49 (2H, m), 10.96 (1H, s), 13.86 (1H, br s).
References:
EP2123652,2009,A1 Location in patent:Page/Page column 44
6602-54-6
511 suppliers
$6.00/10g
![2-((tert-butoxycarbonyl)amino)thieno[2,3-b]pyridine-3-carboxylic acid](/CAS/20180703/GIF/1042441-74-6.gif)
1042441-74-6
4 suppliers
inquiry
![3-BroMothieno[2,3-b]pyridine-2-carboxylic acid](/CAS/GIF/72832-25-8.gif)
72832-25-8
13 suppliers
inquiry
![2-((tert-butoxycarbonyl)amino)thieno[2,3-b]pyridine-3-carboxylic acid](/CAS/20180703/GIF/1042441-74-6.gif)
1042441-74-6
4 suppliers
inquiry
![ETHYL 3-AMINOTHIENO[2,3-B]PYRIDINE-2-CARBOXYLATE](/CAS/GIF/52505-46-1.gif)
52505-46-1
97 suppliers
$20.00/1g
![2-((tert-butoxycarbonyl)amino)thieno[2,3-b]pyridine-3-carboxylic acid](/CAS/20180703/GIF/1042441-74-6.gif)
1042441-74-6
4 suppliers
inquiry