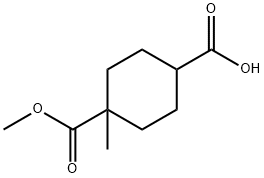
4-(methoxycarbonyl)-4-methylcyclohexanecarboxylic acid synthesis
- Product Name:4-(methoxycarbonyl)-4-methylcyclohexanecarboxylic acid
- CAS Number:1056639-33-8
- Molecular formula:C10H16O4
- Molecular Weight:200.23
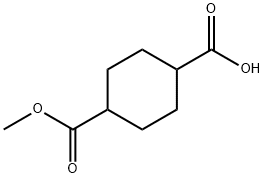
32529-79-6
75 suppliers
$6.00/250mg

74-88-4
340 suppliers
$15.00/10g

1056639-33-8
16 suppliers
$45.00/5mg
Yield:-
Reaction Conditions:
Stage #1: 4-(methoxycarbonyl)cyclohexanecarboxylic acidwith lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 0.916667 h;
Stage #2: methyl iodide in tetrahydrofuran;n-heptane;ethylbenzene at -78 - 20; for 4.75833 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;n-heptane;ethylbenzene;water at 0;
Steps:
1
INTERMEDIATE 39: methyl 4- [1 -hydroxy- 1-( 1,3 -thiazol-2-yl)ethyl]-l-methylcyclohexane- carboxylateStep 1 : To a solution of lithium diisopropyl amide (1.8 M in tetrahydrofuran/ heptane/ ethylbenzene, 46.2 mL, 83 mmol) at -78 °C was added a solution of 4-(methoxycarbonyl)- cyclohexanecarboxylic acid (6.2 g, 33.3 mmol) in tetrahydrofuran (80 mL) over 15 minutes via cannula needle. After 40 minutes of stirring, iodomethane (3.12 mL, 49.9 mmol) was added over 30 seconds to the brown reaction mixture. After 45 minutes the reaction bath was allowed to warm to room temperature over four hours, then the reaction mixture was cooled to 0 °C before hydrochloric acid (2.0 M in water, 100 mL) was added. The reaction mixture was diluted with hexanes (50 mL) and the layers were separated. The aqueous layer was extracted with ethyl acetate (2xl00mL). The combined organic layers were washed with brine (3x50 mL), dried over sodium sulfate, filtered, and concentrated to give 4-(methoxycarbonyl)-4-methylcyclohexane- carboxylic acid, which was used without further purification.
References:
WO2012/154519,2012,A1 Location in patent:Page/Page column 62-63
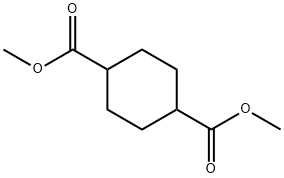
94-60-0
216 suppliers
$6.00/10g

1056639-33-8
16 suppliers
$45.00/5mg