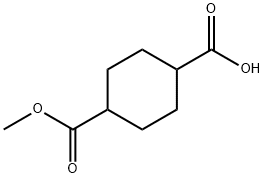
4-CARBOMETHOXY-CYCLOHEXANE-1-CARBOXYLIC ACID synthesis
- Product Name:4-CARBOMETHOXY-CYCLOHEXANE-1-CARBOXYLIC ACID
- CAS Number:32529-79-6
- Molecular formula:C9H14O4
- Molecular Weight:186.21
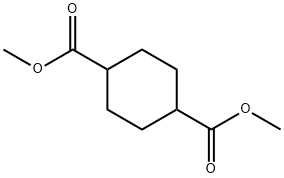
94-60-0

32529-79-6
Step 1. Preparation of 4-(methoxycarbonyl)cyclohexanecarboxylic acid (i-12b). Dimethyl cyclohexane-1,4-dicarboxylate (i-12a) (8 g, 40 mmol) was reacted with barium hydroxide (6.3 g, 20 mmol) in 80% aqueous methanol (150 mL) for 12 h at 25 °C with stirring. Upon completion of the reaction, the mixture was diluted with water (200 mL) and washed with hexane (100 mL x 2) to remove unreacted starting materials. Subsequently, the aqueous layer was acidified to pH=3 with 2M HCl solution and extracted with ethyl acetate (EtOAc) (100mL x 3). The organic layers were combined, washed with water (100 mL), dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent ratio: petroleum ether (PE):ethyl acetate (EtOAc) = 50:1 to 3:1) to afford 4-(methoxycarbonyl)cyclohexanecarboxylic acid (3.2 g, 43% yield) as a white solid.LC-MS (ESI) analysis: calculated value of C9H14O4 [M+H]+ was 187, and the measured value was 187.

94-60-0
243 suppliers
$6.00/10g

32529-79-6
82 suppliers
$6.00/250mg
Yield: 43%
Reaction Conditions:
with water;barium(II) hydroxide in methanol at 25; for 12 h;
Steps:
i.12.1 Step 1. Preparation of 4-(methoxycarbonyl)cyclohexanecarboxylic acid (i-12b).
Step 1. Preparation of 4-(methoxycarbonyl)cyclohexanecarboxylic acid (i-12b).A mixture of dimethyl cyclohexane-1,4-dicarboxylate (i-12a) (8 g, 40 mmol) and bariumhydroxide (6.3 g, 2Ommol) in 80% aqueous methanol (150 mL) was stirred at 25 °C for 12h.The mixture was diluted with water (200 mL) and washed with hexane (100 mLx2) to removeremaining starting material. The aqueous layer was then acidified with 2 M HC1 to pH = 3and extracted with EtOAc (100 mL x 3). The organic layer was washed with water (100 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The mixture was purified by column chromatography on silica gel (PE: EtOAc = 50:1 to 3:1) to afford the title compound (3.2 g, 43%) as a white solid. LCMS (ESI): calc’d for C9H1404 [M+H]: 187,found: 187;
References:
Location in patent:Page/Page column 54
1679-64-7
346 suppliers
$5.00/5g

32529-79-6
82 suppliers
$6.00/250mg