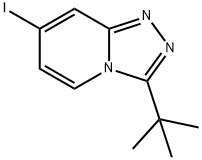
3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine synthesis
- Product Name:3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine
- CAS Number:1057393-45-9
- Molecular formula:C10H12IN3
- Molecular Weight:301.13
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine](/CAS/20180703/GIF/1057393-45-9.gif)
1057393-45-9
5 suppliers
inquiry
Yield: 57%
Reaction Conditions:
Stage #1:N'-(4-iodopyridin-2-yl)-2,2-dimethylpropanohydrazide with trichlorophosphate at 100; for 0.833333 h;
Stage #2: with sodium hydroxide in water
Steps:
2.b
The solid obtained in step a) was reacted with phosphorous oxychloride at 1000C for 50 minutes, then the reaction was poured into ice, neutralized with sodium hydroxide 8M and extracted with ethyl acetate (500 ml_). The organic phase was washed with aqueous sodium bicarbonate (4%), water and brine and it was dried over anhydrous sodium sulphate and the solvent removed under reduced pressure. The resulting residue was triturated with hexanes/diethyl ether to afford 6.72 g (57%) of the desired product. Variable amounts of the 7-chloro analogue were observed (approx. 10%) but the crude product obtained was used in the next step without further purification.LRMS (m/z): 302 (M+1)+.1H-NMR δ (CDCI3): 1.60 (s, 9H), 7.00 (d, J=7.3 Hz1 1 H), 7.93 (d, J=7.3 Hz, 1 H), 8.21 (s, 1 H).
References:
LABORATORIOS ALMIRALL, S.A. WO2008/107125, 2008, A1 Location in patent:Page/Page column 35
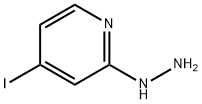
1057393-44-8
31 suppliers
$59.00/500mg
![3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine](/CAS/20180703/GIF/1057393-45-9.gif)
1057393-45-9
5 suppliers
inquiry
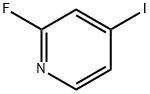
22282-70-8
274 suppliers
$8.00/1g
![3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine](/CAS/20180703/GIF/1057393-45-9.gif)
1057393-45-9
5 suppliers
inquiry
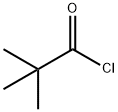
3282-30-2
380 suppliers
$18.00/100ml
![3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine](/CAS/20180703/GIF/1057393-45-9.gif)
1057393-45-9
5 suppliers
inquiry
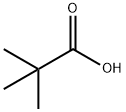
75-98-9
437 suppliers
$10.00/10g
![3-(tert-Butyl)-7-iodo-[1,2,4]triazolo[4,3-a]pyridine](/CAS/20180703/GIF/1057393-45-9.gif)
1057393-45-9
5 suppliers
inquiry