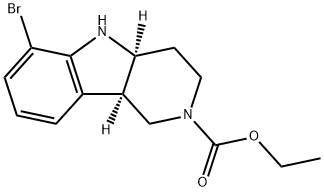
ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate synthesis
- Product Name:ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate
- CAS Number:1059630-08-8
- Molecular formula:C14H17BrN2O2
- Molecular Weight:325.2
![(4aS,9bR)-6-bromo-1H,2H,3H,4H,4aH,5H,9bH-pyrido[4,3-b]indole](/CAS/20180713/GIF/1059630-13-5.gif)
1059630-13-5
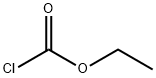
541-41-3
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
Example 3: Preparation of ethyl (4aS,9bR)-6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate Step 1: (4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole (36.0 g, 0.142 mol) was dissolved in 50% aqueous sodium hydroxide to free the base, followed by extraction of the product with methyl tert-butyl ether (MTBE). Step 2: The above free base (36.0g, 0.142mol) was dissolved in THF (300mL), triethylamine (24mL) was added and cooled in an ice water bath. Ethyl chloroformate (13.5mL, 0.142mol) was slowly added dropwise over 1 hour using a syringe pump. After removing the ice water bath, the reaction mixture was continued to be stirred at room temperature for 1 hour. Upon completion of the reaction, it was filtered through a diatomaceous earth pad and the solvent was evaporated to afford ethyl (4aS,9bR)-6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate. 1H NMR (CDCl3, 300MHz): δ 1.20-1.35 (m, 3H), 1.73-1.85 (m, 1H), 1.85-1.99 (m, 1H), 3.22-3.52 (m, 3H), 3.52-3.66 (m, 1H), 3.66-3.95 (br, 1H), 3.95-4.21 (m, 4H), 6.60 (m, 4H). 4H), 6.60 (t, J = 7.7 Hz, 1H), 7.04 (d, J = 7.2 Hz, 1H), 7.20 (d, J = 8.1 Hz, 1H). Alternative method: the (S)-mandelate of (4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole was used as starting material. To a 100 mL round bottom flask was added (S)-mandelate feedstock (5 g, 12.35 mmol), Na2CO3 (2.88 g, 27.17 mmol) and THF (25 mL). A solution of ethyl chloroformate (1.64 g, 15.11 mmol) in THF (5 mL) was added dropwise over 25 min at 25 °C. After the reaction mixture was stirred at 25 °C for 10 min, detection by HPLC showed less than 2% of starting material remaining and a product yield of about 98%. EtOH (12.5 mL) was added and about 30 mL of solvent (mostly THF) was removed by concentration under reduced pressure. H2O (37.5 mL) was added and the mixture was pH > 9. After stirring at room temperature for 1 hr, the product was filtered, the solid was washed with H2O (25 mL) and dried under vacuum at 58 °C for 16 hr to give a yellow solid of 3.9442 g (98% yield).1H NMR was consistent with the target product, and (S)-mandelic acid was not detected. hplc analysis showed a purity of the product of > 99%. lc-ms showed molecular ion peak M/e = 326 (M + 1).
![(4aS,9bR)-6-bromo-1H,2H,3H,4H,4aH,5H,9bH-pyrido[4,3-b]indole](/CAS/20180713/GIF/1059630-13-5.gif)
1059630-13-5
61 suppliers
$45.00/50mg

541-41-3
0 suppliers
$21.00/5g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
63 suppliers
$38.00/100mg
Yield:1059630-08-8 98%
Reaction Conditions:
with sodium carbonate in tetrahydrofuran at 25; for 1.33333 h;Product distribution / selectivity;
Steps:
3
Example 3: Production of (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-lH- pyrido[4,3-b]indoIe-2(9bH)-carboxylate; (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro- 1 H-pyrido[4,3-b]indole-2(9bH)-carboxylate may be prepared by first optaining [4aS, 9bR]-6-bromo- 2,3,4,4a,5,9b-hexahydro-lH-pyrido[4,3-Z>]indole (36.0 g, 0.142mol)) as a free base by using 50% aqueous sodium hydroxide solution and extracting the product into MTBE. The conversion to (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-lη-pyrido[4,3- b]indole-2(9bH)-carboxylate may then be done by cooling a suspension of compounds of [4aS, 9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-lH-pyrido[4,3-*]indole (36.0 g, 0.142mol)) in THF (300 ml) and triethylamine (24 ml) in an ice- water bath. Ethyl chloroformate is added dropwise (13.5 ml, 0.142mol) via a syringe pump over 1 hour. The ice-water bath is removed and the reaction mixture is stirred at room temperature for another hour. The reaction mixture is passed through a pad of celite and the solvent is evaporated to give (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-lH-pyrido[4,3- b]indole-2(9bH)-carboxylate). 1H NMR (CDCl3, 300 MHz): 1.20-1.35 (m,3H), 1.73- 1.85 (m, IH), 1.85-1.99 (m, IH), 3.22-3.52 (m, 3H), 3.52-3.66 (m, IH), 3.66-3.95 (Br, IH), 3.95-4.21 (m, 4H), 6.60 (t, J = 7.7 Hz, IH), 7.04 (d, J = 7.2 Hz, IH), 7.20 (d, J = 8.1 Hz, IH).[0084] Alternative to the use of [4aS, 9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro- lH-pyrido[4,3-Z>]indole (Compound of Formual 1C) free base, the reaction may also be done by starting with the (S)-mandelate salt of [4aS, 9bR]-6-bromo-2,3,4,4a,5,9b-
References:
WO2008/112280,2008,A1 Location in patent:Page/Page column 85-86
![(4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole](/CAS/20200119/GIF/1059630-07-7.gif)
1059630-07-7
62 suppliers
inquiry

541-41-3
0 suppliers
$21.00/5g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
63 suppliers
$38.00/100mg
![1H-Pyrido[4,3-b]indole, 6-bromo-2,3,4,5-tetrahydro-, hydrochloride (1:1)](/CAS/20211123/GIF/1059630-11-3.gif)
1059630-11-3
38 suppliers
$90.00/50mg
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
63 suppliers
$38.00/100mg
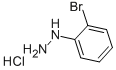
50709-33-6
294 suppliers
$5.00/1g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
63 suppliers
$38.00/100mg
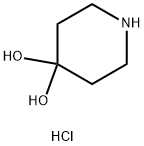
40064-34-4
0 suppliers
$10.00/5g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
63 suppliers
$38.00/100mg