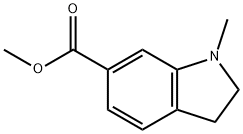
Methyl 1-Methyl-2,3-dihydro-1H-indole-6-carboxylate synthesis
- Product Name:Methyl 1-Methyl-2,3-dihydro-1H-indole-6-carboxylate
- CAS Number:1071432-28-4
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
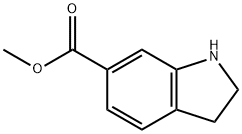
341988-36-1
112 suppliers
$50.00/100mg

74-88-4
356 suppliers
$15.00/10g

1071432-28-4
6 suppliers
inquiry
Yield:1071432-28-4 47%
Reaction Conditions:
Stage #1: 2,3-dihydro-1H-indole-6-carboxylic acid methyl esterwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.166667 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 1 h;
Steps:
2
A solution of methyl indoline-6-carboxylate (200 mg, 1.13 mmol) in N,N- dimethylformamide (3 ml.) was cooled to 0 °C in an ice-water bath before the addition of sodium hydride (50 mg, 1.24 mmol). The reaction mixture was stirred for 10 minutes before iodomethane (0.08 ml_, 1.24 mmol) was added dropwise. The reaction mixture was warmed to room temperature and stirred for 1 hour. After this time the reaction mixture was diluted with saturated NaHCCh solution and extracted with ethyl acetate. The organics were dried over anhydrous MgS04, filtered and concentrated under reduced pressure. Purification by column chromatography (0-50% ethyl acetate in cyclohexane) gave methyl 1 - methylindoline-6-carboxylate (102 mg, 0.53 mmol, 47%) as a pale yellow oil. LCMS: MS m/z 192.1 [M+H]+.
References:
WO2020/43866,2020,A1 Location in patent:Page/Page column 88; 87
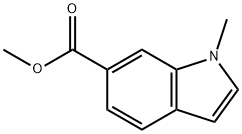
1204-32-6
77 suppliers
$10.00/250mg

1071432-28-4
6 suppliers
inquiry
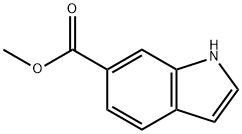
50820-65-0
254 suppliers
$6.00/1g

1071432-28-4
6 suppliers
inquiry